Page 365 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 365
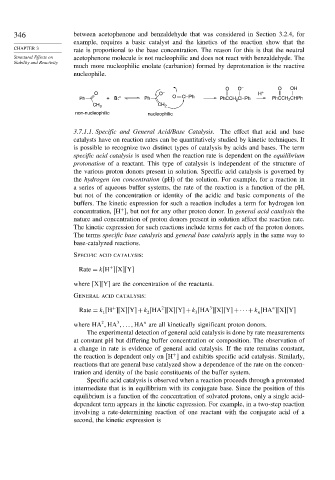
346 between acetophenone and benzaldehyde that was considered in Section 3.2.4, for
example, requires a basic catalyst and the kinetics of the reaction show that the
CHAPTER 3 rate is proportional to the base concentration. The reason for this is that the neutral
Structural Effects on acetophenone molecule is not nucleophilic and does not react with benzaldehyde. The
Stability and Reactivity
much more nucleophilic enolate (carbanion) formed by deprotonation is the reactive
nucleophile.
O O – O OH
O O – H +
Ph + B: – Ph O CHPh PhCCH 2 CHPh PhCCH 2 CHPh
CH 3 CH 2
non-nucleophilic nucleophilic
3.7.1.1. Specific and General Acid/Base Catalysis. The effect that acid and base
catalysts have on reaction rates can be quantitatively studied by kinetic techniques. It
is possible to recognize two distinct types of catalysis by acids and bases. The term
specific acid catalysis is used when the reaction rate is dependent on the equilibrium
protonation of a reactant. This type of catalysis is independent of the structure of
the various proton donors present in solution. Specific acid catalysis is governed by
the hydrogen ion concentration (pH) of the solution. For example, for a reaction in
a series of aqueous buffer systems, the rate of the reaction is a function of the pH,
but not of the concentration or identity of the acidic and basic components of the
buffers. The kinetic expression for such a reaction includes a term for hydrogen ion
+
concentration, H , but not for any other proton donor. In general acid catalysis the
nature and concentration of proton donors present in solution affect the reaction rate.
The kinetic expression for such reactions include terms for each of the proton donors.
The terms specific base catalysis and general base catalysis apply in the same way to
base-catalyzed reactions.
Specific acid catalysis:
Rate = k H X Y
+
where [X][Y] are the concentration of the reactants.
General acid catalysis:
n
3
2
Rate = k H X Y +k HA X Y +k HA X Y +···+k HA X Y
+
1 2 3 n
n
3
2
where HA HA HA are all kinetically significant proton donors.
The experimental detection of general acid catalysis is done by rate measurements
at constant pH but differing buffer concentration or composition. The observation of
a change in rate is evidence of general acid catalysis. If the rate remains constant,
+
the reaction is dependent only on [H ] and exhibits specific acid catalysis. Similarly,
reactions that are general base catalyzed show a dependence of the rate on the concen-
tration and identity of the basic constituents of the buffer system.
Specific acid catalysis is observed when a reaction proceeds through a protonated
intermediate that is in equilibrium with its conjugate base. Since the position of this
equilibrium is a function of the concentration of solvated protons, only a single acid-
dependent term appears in the kinetic expression. For example, in a two-step reaction
involving a rate-determining reaction of one reactant with the conjugate acid of a
second, the kinetic expression is