Page 367 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 367
348 3.7.1.2. Brønsted Catalysis Law. As might be expected, there is a relationship
between the effectiveness of general acid catalysts and the acidic strength of a proton
CHAPTER 3 donor, as measured by its acid dissociation constant K . The stronger acids are more
a
Structural Effects on effective catalysts. This relationship is expressed by the Brønsted catalysis law:
Stability and Reactivity
logk cat = logK +b (3.51)
An analogous equation holds for catalysis by bases. This equation requires that the E
a
for the catalytic step for a series of acids be directly proportional to G of dissociation
for the same series of acids. The proportionality constant is an indication of the
sensitivity of the catalytic step to structural changes, relative to the effect of the same
structural changes on acid dissociation. It is often found that a single proportionality
constant is restricted to only structurally similar acids and that linear relationships
having values of different magnitude apply to each type of acid.
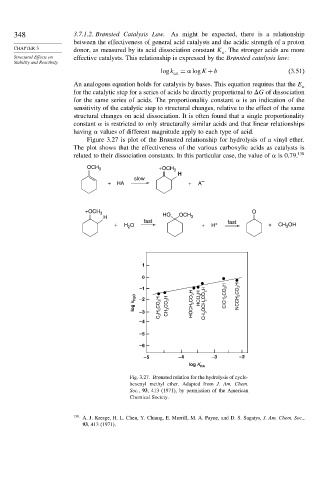
Figure 3.27 is plot of the Brønsted relationship for hydrolysis of a vinyl ether.
The plot shows that the effectiveness of the various carboxylic acids as catalysts is
related to their dissociation constants. In this particular case, the value of is 0.79. 138
OCH 3 +OCH 3
H
slow
+ HA + A –
+OCH 3 O
H HO OCH 3
fast fast
+ H O + H + + CH OH
3
2
1
0
–1
log k H 2 O – 2 CH 3 CO 2 H HOCH 2 CO 2 H HCO 2 H CH 3 OCH 2 CO 2 H ClCH 2 CO 2 H NCCH 2 CO 2 H
– 3 C 2 H 5 CO 2 H
– 4
– 5
– 6
– 5 – 4 – 3 – 2
log K HA
Fig. 3.27. Brønsted relation for the hydrolysis of cyclo-
hexenyl methyl ether. Adapted from J. Am. Chem.
Soc., 93, 413 (1971), by permission of the American
Chemical Society.
138
A. J. Kresge, H. L. Chen, Y. Chiang, E. Murrill, M. A. Payne, and D. S. Sagatys, J. Am. Chem. Soc.,
93, 413 (1971).