Page 372 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 372
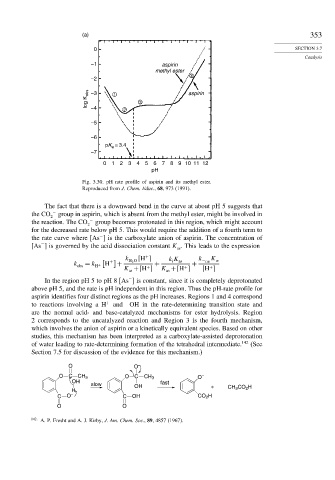
(a) 353
0 SECTION 3.7
Catalysis
–1 aspirin
methyl ester
4
–2
log K obs –3 1 2 3 aspirin
–4
–5
–6
pK = 3.4
a
–7
0 1 2 3 4 5 6 7 8 9 10 11 12
pH
Fig. 3.30. pH rate profile of aspirin and its methyl ester.
Reproduced from J. Chem. Educ., 68, 973 (1991).
The fact that there is a downward bend in the curve at about pH 5 suggests that
the CO − group in aspirin, which is absent from the methyl ester, might be involved in
2
the reaction. The CO − group becomes protonated in this region, which might account
2
for the decreased rate below pH 5. This would require the addition of a fourth term to
−
the rate curve where As is the carboxylate anion of aspirin. The concentration of
−
As is governed by the acid dissociation constant K . This leads to the expression
as
+
k H k K k K
H 2 O i as − OH w
k = k + H + + + +
obs H
K + H K + H H
+
+
+
as
as
In the region pH 5 to pH 8 As is constant, since it is completely deprotonated
−
above pH 5, and the rate is pH independent in this region. Thus the pH-rate profile for
aspirin identifies four distinct regions as the pH increases. Regions 1 and 4 correspond
to reactions involving a H + and − OH in the rate-determining transition state and
are the normal acid- and base-catalyzed mechanisms for ester hydrolysis. Region
2 corresponds to the uncatalyzed reaction and Region 3 is the fourth mechanism,
which involves the anion of aspirin or a kinetically equivalent species. Based on other
studies, this mechanism has been interpreted as a carboxylate-assisted deprotonation
of water leading to rate-determining formation of the tetrahedral intermediate. 142 (See
Section 7.5 for discussion of the evidence for this mechanism.)
O O –
O C CH 3 O C CH 3 O –
OH fast
slow OH + CH 3 CO 2 H
H
C O – C OH CO 2 H
O O
142
A. P. Fresht and A. J. Kirby, J. Am. Chem. Soc., 89, 4857 (1967).