Page 375 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 375
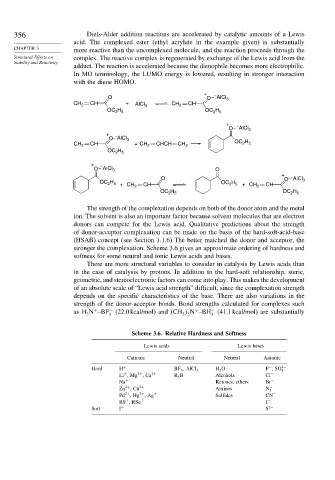
356 Diels-Alder addition reactions are accelerated by catalytic amounts of a Lewis
acid. The complexed ester (ethyl acrylate in the example given) is substantially
CHAPTER 3 more reactive than the uncomplexed molecule, and the reaction proceeds through the
Structural Effects on complex. The reactive complex is regenerated by exchange of the Lewis acid from the
Stability and Reactivity
adduct. The reaction is accelerated because the dienophile becomes more electrophilic.
In MO terminology, the LUMO energy is lowered, resulting in stronger interaction
with the diene HOMO.
+
O O – AlCl 3
CH 2 CH + AlCl 3 CH 2 CH
H
OC H OC 2 5
2 5
+ –
O AlCl 3
+ –
O AlCl 3
CH + CH CHCH CH OC H
2 5
CH 2 2 2
OC H
2 5
+ –
O AlCl 3 O
+
O O – AlCl
OC H + CH 2 CH OC H + CH 2 CH 3
2 5
2 5
H
OC 2 H 5 OC 2 5
The strength of the complexation depends on both of the donor atom and the metal
ion. The solvent is also an important factor because solvent molecules that are electron
donors can compete for the Lewis acid. Qualitative predictions about the strength
of donor-acceptor complexation can be made on the basis of the hard-soft-acid-base
(HSAB) concept (see Section 1.1.6) The better matched the donor and acceptor, the
stronger the complexation. Scheme 3.6 gives an approximate ordering of hardness and
softness for some neutral and ionic Lewis acids and bases.
There are more structural variables to consider in catalysis by Lewis acids than
in the case of catalysis by protons. In addition to the hard-soft relationship, steric,
geometric, and stereoelectronic factors can come into play. This makes the development
of an absolute scale of “Lewis acid strength” difficult, since the complexation strength
depends on the specific characteristics of the base. There are also variations in the
strength of the donor-acceptor bonds. Bond strengths calculated for complexes such
−
+
+
as H N –BF (22.0 kcal/mol) and CH N –BH (41.1 kcal/mol) are substantially
−
3
3 3
3
3
Scheme 3.6. Relative Hardness and Softness
Lewis acids Lewis bases
Cationic Neutral Neutral Anionic
Hard H + BF 3 , AlCl 3 H 2 O F ,SO 2−
−
4
+
Li ,Mg ,Ca 2+ R 3 B Alcohols Cl −
2+
Na + Ketones, ethers Br −
Zn ,Cu 2+ Amines N −
2+
3
2+
Pd ,Hg ,Ag + Sulfides CN −
2+
RS , RSe + I −
+
Soft I + S 2−