Page 380 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 380
As a specific example of a solvent effect, let us consider how the solvent affects 361
the solvolysis of t-butyl chloride. Much evidence, which is discussed in detail in
Chapter 4, indicates that the rate-determining step of the reaction is ionization of the SECTION 3.8
carbon-chlorine bond to give a carbocation and the chloride ion. The TS reflects some Solvent Effects
of the charge separation that occurs in the ionization:
+
−
+
CH C−Cl → CH C ------Cl → CH C +Cl −
3 3
3 3
3 3
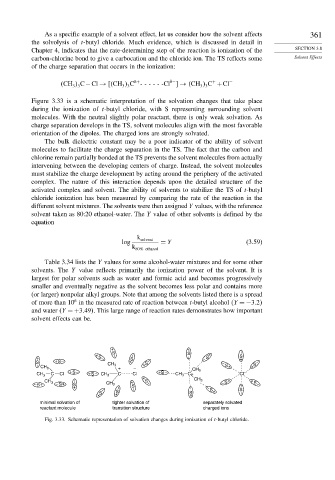
Figure 3.33 is a schematic interpretation of the solvation changes that take place
during the ionization of t-butyl chloride, with S representing surrounding solvent
molecules. With the neutral slightly polar reactant, there is only weak solvation. As
charge separation develops in the TS, solvent molecules align with the most favorable
orientation of the dipoles. The charged ions are strongly solvated.
The bulk dielectric constant may be a poor indicator of the ability of solvent
molecules to facilitate the charge separation in the TS. The fact that the carbon and
chlorine remain partially bonded at the TS prevents the solvent molecules from actually
intervening between the developing centers of charge. Instead, the solvent molecules
must stabilize the charge development by acting around the periphery of the activated
complex. The nature of this interaction depends upon the detailed structure of the
activated complex and solvent. The ability of solvents to stabilize the TS of t-butyl
chloride ionization has been measured by comparing the rate of the reaction in the
different solvent mixtures. The solvents were then assigned Y values, with the reference
solvent taken as 80:20 ethanol-water. The Y value of other solvents is defined by the
equation
k solvent
log = Y (3.59)
k 80% ethanol
Table 3.34 lists the Y values for some alcohol-water mixtures and for some other
solvents. The Y value reflects primarily the ionization power of the solvent. It is
largest for polar solvents such as water and formic acid and becomes progressively
smaller and eventually negative as the solvent becomes less polar and contains more
(or larger) nonpolar alkyl groups. Note that among the solvents listed there is a spread
6
of more than 10 in the measured rate of reaction between t-butyl alcohol Y =−3 2
and water Y =+3 49 . This large range of reaction rates demonstrates how important
solvent effects can be.
+ + – + S + S –
S –
S – S – S +
S – + S – – –
+
+
CH 3 + – S
CH 3 S + +
+ – CH 3
S –
C Cl + S – S C Cl + S – CH 3 C+ Cl –
CH 3 + – CH 3
+
CH 3 +
CH 3 – + S +
S + – S + – + S CH 3 – S – S – – – – + S –
S – S S S + S –
+ + +
minimal solvation of tighter solvation of separately solvated
reactant molecule transition structure charged ions
Fig. 3.33. Schematic representation of solvation changes during ionization of t-butyl chloride.