Page 384 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 384
an organic reactant, typically in a hydrocarbon or nonpolar halogenated solvent. The 365
addition of phase transfer catalysts causes migration of the anion into the organic
phase and, because of its high nucleophilicity, reaction occurs under exceptionally SECTION 3.8
mild conditions. Solvent Effects
+
R N R N +
4
4
X – X –
(s) (s)
Nu: – Nu: –
M + M +
3.8.2.3. Differential Solvation of Reactants and Transition States. It should always
be kept in mind that solvent effects can modify the energy of both the reactants and
the transition state. It is the difference in the solvation that is the basis for changes in
activation energies and reaction rates. Thus, although it is common to discuss solvent
effects solely in terms of reactant solvation or transition state solvation, this is an
oversimplification. A case that illustrates this point is the base-promoted hydrolysis of
esters by hydroxide ion.
O O –
O
COC H HOC H
CH 3 2 5 CH 3 C OC H CH 3 C + 2 5
2 5
O –
–
OH OH
Reactants Transition structure Products
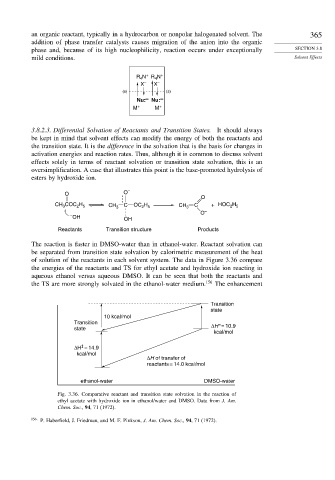
The reaction is faster in DMSO-water than in ethanol-water. Reactant solvation can
be separated from transition state solvation by calorimetric measurement of the heat
of solution of the reactants in each solvent system. The data in Figure 3.36 compare
the energies of the reactants and TS for ethyl acetate and hydroxide ion reacting in
aqueous ethanol versus aqueous DMSO. It can be seen that both the reactants and
the TS are more strongly solvated in the ethanol-water medium. 156 The enhancement
Transition
state
10 kcal/mol
Transition
ΔH* = 10.9
state
kcal/mol
‡
ΔH = 14.9
kcal/mol
ΔH of transfer of
reactants = 14.0 kcal/mol
ethanol-water DMSO-water
Fig. 3.36. Comparative reactant and transition state solvation in the reaction of
ethyl acetate with hydroxide ion in ethanol/water and DMSO. Data from J. Am.
Chem. Soc., 94, 71 (1972).
156
P. Haberfield, J. Friedman, and M. F. Pinkson, J. Am. Chem. Soc., 94, 71 (1972).