Page 386 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 386
– +
O K OC H O 367
2 5
CH C CHCO C H + C H X CH C CHCO C H + CH 3 CCHCO C H SECTION 3.8
3
2 2 5
3
2 2 5
2 2 5
2 5
C H Solvent Effects
2 5
X = OTs 88% 11% (plus 1% dialkyl)
X = Cl 60% 32% (plus 8% dialkyl)
X = Br 39% 38% (plus 23% dialkyl)
X = I 13% 71% (plus 16% dialkyl)
Leaving-group effects on the ratio of C- to O-alkylation can be correlated by the
HSAB rationale. 159 Of the two nucleophilic sites in an enolate ion, oxygen is harder
than carbon. Nucleophilic substitution reactions of the S 2 type proceed best when the
N
nucleophile and leaving group are either both hard or both soft. 160 Consequently, ethyl
iodide, with the soft leaving group iodide, reacts preferentially with the softer carbon
site rather than the harder oxygen. Oxygen-leaving groups, such as sulfonate and
sulfate, are harder and react preferentially at the hard oxygen site of the enolate. The
hard-hard combination is favored by an early transition state where the electrostatic
attraction is the most important factor. The soft-soft combination is favored by a later
transition state where partial bond formation is the dominant factor. The C-alkylation
product is more stable than the O-alkylation product (because the bond energy of
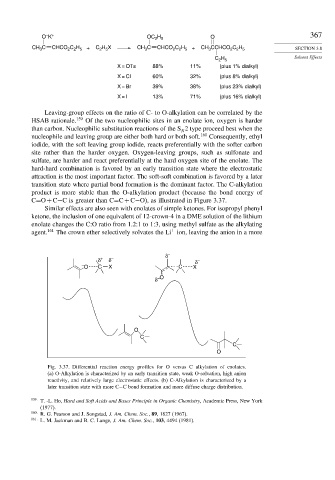
C=O+C−C is greater than C=C+C−O), as illustrated in Figure 3.37.
Similar effects are also seen with enolates of simple ketones. For isopropyl phenyl
ketone, the inclusion of one equivalent of 12-crown-4 in a DME solution of the lithium
enolate changes the C:O ratio from 1.2:1 to 1:3, using methyl sulfate as the alkylating
+
agent. 161 The crown ether selectively solvates the Li ion, leaving the anion in a more
δ –
δ + δ – δ –
– O C X C X
δ – O
O
C
C
O
Fig. 3.37. Differential reaction energy profiles for O versus C alkylation of enolates.
(a) O-Alkylation is characterized by an early transition state, weak O-solvation, high anion
reactivity, and relatively large electrostatic effects. (b) C-Alkylation is characterized by a
later transition state with more C−C bond formation and more diffuse charge distribution.
159 T. -L. Ho, Hard and Soft Acids and Bases Principle in Organic Chemistry, Academic Press, New York
(1977).
160 R. G. Pearson and J. Songstad, J. Am. Chem. Soc., 89, 1827 (1967).
161
L. M. Jackman and B. C. Lange, J. Am. Chem. Soc., 103, 4494 (1981).