Page 382 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 382
general theory for quantitatively predicting such specific effects has been developed 363
to date. Nevertheless, there are many cases where specific interactions with solvent
molecules strongly influence the outcome and/or the rate of reactions. SECTION 3.8
Solvent Effects
3.8.2.1. Enhanced Nucleophilicity in Polar Aprotic Solvents. An important example
of solvent effects is the enhanced nucleophilicity of many anions in polar aprotic
solvents as compared with protic solvents. 153 In protic solvents, anions are strongly
solvated by hydrogen bonding. This is particularly true for hard anions that have a
high concentration of charge on oxygen such as alkoxide ions. Hydrogen-bonding
decreases the ability of the nucleophile to act as an electron donor. Stated another
way, the energy required to disrupt hydrogen bonding adds to the activation energy of
the reaction.
In polar aprotic solvents, no hydrogens suitable for hydrogen bonding are present.
As a result, the electrons of the anion are more available for reaction. The anion is at
a higher energy level because of the small stabilization by the solvent. The polarity of
the aprotic solvents is also important for the solubility of ionic compounds. Dissolved
ionic compounds are likely to be present as ion pairs or larger aggregates in which the
reactivity of the anion is diminished by the electrostatic interaction with the cation.
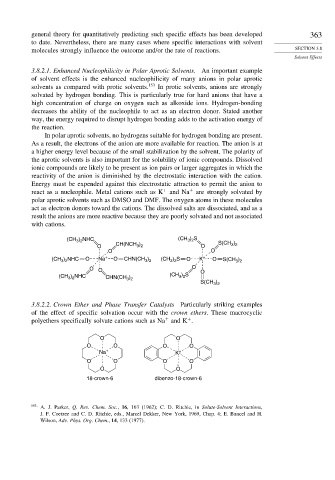
Energy must be expended against this electrostatic attraction to permit the anion to
+
react as a nucleophile. Metal cations such as K and Na are strongly solvated by
+
polar aprotic solvents such as DMSO and DMF. The oxygen atoms in these molecules
act as electron donors toward the cations. The dissolved salts are dissociated, and as a
result the anions are more reactive because they are poorly solvated and not associated
with cations.
(CH ) NHC (CH ) S
3 2
3 2
3 2
3 2
O CH(NCH ) O S(CH )
O O
) NHC O Na + O CHN(CH ) (CH ) S O K + O
)
(CH 3 2 3 2 3 2 S(CH 3 2
O O O O
) NHC
3 2
(CH 3 2 CHN(CH ) (CH ) S
3 2
S(CH )
3 2
3.8.2.2. Crown Ether and Phase Transfer Catalysts Particularly striking examples
of the effect of specific solvation occur with the crown ethers. These macrocyclic
+
+
polyethers specifically solvate cations such as Na and K .
O O
O O O O
Na + K +
O O O O
O O
18-crown-6 dibenzo-18-crown-6
153
A. J. Parker, Q. Rev. Chem. Soc., 16, 163 (1962); C. D. Ritchie, in Solute-Solvent Interactions,
J. F. Coetzee and C. D. Ritchie, eds., Marcel Dekker, New York, 1969, Chap. 4; E. Buncel and H.
Wilson, Adv. Phys. Org. Chem., 14, 133 (1977).