Page 377 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 377
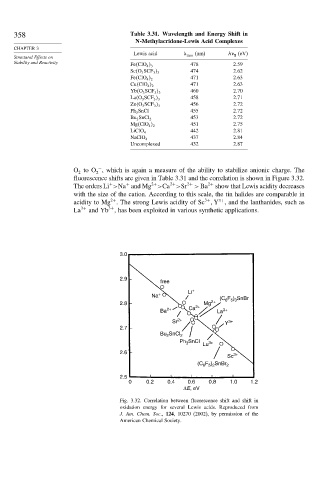
358 Table 3.31. Wavelength and Energy Shift in
N-Methylacridone-Lewis Acid Complexes
CHAPTER 3
Lewis acid max (nm) h fl (eV)
Structural Effects on
Stability and Reactivity
Fe ClO 4 3 478 2 59
474 2 62
Sc O 3 SCF 3 3
471 2 63
Fe ClO 4 2
471 2 63
Cu ClO 4 2
460 2 70
Yb O 3 SCF 3 3
458 2 71
La O 3 SCF 3 3
456 2 72
Zn O 3 SCF 3 3
Ph 3 SnCl 455 2 72
453 2 72
Bu 2 SnCl 2
451 2 75
Mg ClO 4 2
442 2 81
LiClO 4
437 2 84
NaClO 4
Uncomplexed 432 2 87
O to O , which is again a measure of the ability to stabilize anionic charge. The
−
2 2
fluorescence shifts are given in Table 3.31 and the correlation is shown in Figure 3.32.
+
2+
2+
+
The orders Li >Na and Mg >Ca >Sr 2+ > Ba 2+ show that Lewis acidity decreases
with the size of the cation. According to this scale, the tin halides are comparable in
2+
3+
acidity to Mg . The strong Lewis acidity of Sc Y , and the lanthanides, such as
3+
3+
La 3+ and Yb , has been exploited in various synthetic applications.
3.0
2.9
free
Li +
Na + (C F ) SnBr
2.8 2+ Mg 2+ 6 5 3
Ba 2+ Ca La 3+
Sr 2+ Y 3+
2.7
Bu SnCl 2
2
Ph SnCl Lu 3+
3
2.6 3+
Sc
F ) SnBr
(C 6 5 2 2
2.5
0 0.2 0.4 0.6 0.8 1.0 1.2
ΔE, eV
Fig. 3.32. Correlation between fluorescence shift and shift in
oxidation energy for several Lewis acids. Reproduced from
J. Am. Chem. Soc., 124, 10270 (2002), by permission of the
American Chemical Society.