Page 373 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 373
354 3.7.2. Lewis Acid Catalysis
CHAPTER 3 Lewis acids act as electron pair acceptors. The proton is an important special case,
Structural Effects on butmanyothercompoundscatalyzeorganicreactionsbyactingaselectronpairacceptors.
Stability and Reactivity
The most important Lewis acids in organic reactions are metal cations and covalent
compounds of metals. Metal cations that function as Lewis acids include the alkali metal
2+
+
3+
+
+
2+
monocations Li ,Na ,K , di- and trivalent ions such as Mg ,Ca ,Zn ,Sc , and
2+
3+
Bi ; transition metal cations and complexes; and lanthanide cations, such as Ce 3+ and
3+
Yb . Neutral electrophilic covalent molecules can also act as Lewis acids. The most
commonly employed of the covalent compounds include boron trifluoride, aluminum
trichloride, titanium tetrachloride, and tin(IV)tetrachloride. Various other derivatives of
boron, aluminum, titanium, and tin also are Lewis acid catalysts.
The catalytic activity of metal cations originates in the formation of a donor-
acceptor complex between the cation and the reactant, which acts as a Lewis base. As
a result of the complexation, the donor atom becomes effectively more electronegative.
All functional groups that have unshared electron pairs are potential electron donors,
2
but especially prominent in reaction chemistry are carbonyl oxygens sp , hydroxy or
3
ether sp oxygen, as well as similar nitrogen- and sulfur-containing functional groups.
For oxygen and nitrogen functional groups, the catalysis generally correlates with
hardness, so the smaller and more positively charged ions have the strongest effects.
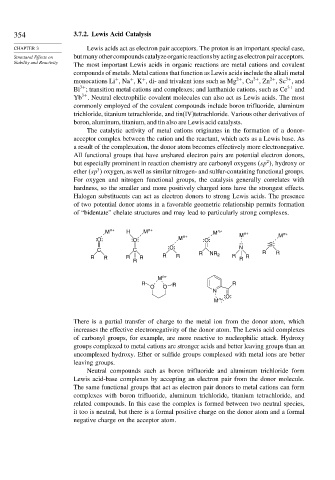
Halogen substituents can act as electron donors to strong Lewis acids. The presence
of two potential donor atoms in a favorable geometric relationship permits formation
of “bidentate” chelate structures and may lead to particularly strong complexes.
M n+ H M n+ M n+ M n+ n+
O O M n+ O M
S
O N
C C R R R
R R R R R R NR 2 R R R
R
M n+
R R R
O O
N
O
M n+
There is a partial transfer of charge to the metal ion from the donor atom, which
increases the effective electronegativity of the donor atom. The Lewis acid complexes
of carbonyl groups, for example, are more reactive to nucleophilic attack. Hydroxy
groups complexed to metal cations are stronger acids and better leaving groups than an
uncomplexed hydroxy. Ether or sulfide groups complexed with metal ions are better
leaving groups.
Neutral compounds such as boron trifluoride and aluminum trichloride form
Lewis acid-base complexes by accepting an electron pair from the donor molecule.
The same functional groups that act as electron pair donors to metal cations can form
complexes with boron trifluoride, aluminum trichloride, titanium tetrachloride, and
related compounds. In this case the complex is formed between two neutral species,
it too is neutral, but there is a formal positive charge on the donor atom and a formal
negative charge on the acceptor atom.