Page 374 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 374
– TiCl – – 355
– – 4 AlCl 3 AlCl 3
BF 3 TiCl 4 +
+ – + O O O O
O BF 3 SECTION 3.7
+ O C R′ C C
C R R C R′ R O + R R N R Catalysis
+
R R R O NR 2 2
Complexes of carbonyl oxygen with trivalent boron and aluminum compounds
tend to adopt a geometry consistent with directional interaction with one of the oxygen
lone pairs. Thus the C−O−M bonds tend to be in the trigonal 120 −140 range
and the boron or aluminum is usually close to the carbonyl plane. 143 The structural
specificity that is built into Lewis acid complexes can be used to advantage to achieve
stereoselectivity in catalysis. For example, use of chiral ligands in conjunction with
Lewis acids is frequently the basis for enantioselective catalysts.
Titanium(IV) tetrachloride and tin(IV) tetrachloride can form complexes that are
similar to those formed by metal ions and those formed by neutral Lewis acids.
Complexation can occur with displacement of a chloride from the metal coordination
sphere or by an increase in the coordination number at the Lewis acid.
– TiCl TiCl 3
+ 4 +
O O O
C + TiCl 4 C C + Cl –
R R R R R R
– SnCl SnCl 3
+ 4 + –
O + SnCl 4 O O + Cl
R R R R R R
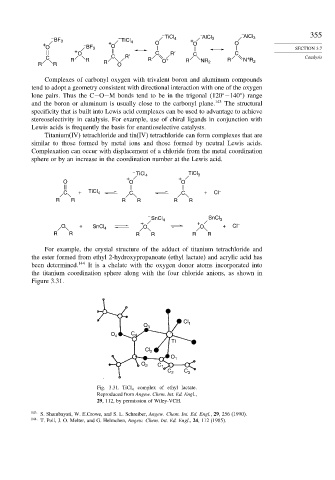
For example, the crystal structure of the adduct of titanium tetrachloride and
the ester formed from ethyl 2-hydroxypropanoate (ethyl lactate) and acrylic acid has
been determined. 144 It is a chelate with the oxygen donor atoms incorporated into
the titanium coordination sphere along with the four chloride anions, as shown in
Figure 3.31.
Cl
O 3 1
O 4 C 4
Ti
Cl 2
O 1
O 2 C 1
C 2 C 3
Fig. 3.31. TiCl 4 complex of ethyl lactate.
Reproduced from Angew. Chem. Int. Ed. Engl.,
29, 112, by permission of Wiley-VCH.
143 S. Shambayati, W. E.Crowe, and S. L. Schreiber, Angew. Chem. Int. Ed. Engl., 29, 256 (1990).
144
T. Poll, J. O. Melter, and G. Helmchen, Angew. Chem. Int. Ed. Engl., 24, 112 (1985).