Page 381 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 381
362 Table 3.34. Y Values for Some Solvent Systems. a
CHAPTER 3 Ethanol-water Y Methanol-water Y Other solvents Y
Structural Effects on 100:0 −2 03 100:0 −1 09 Acetic acid −1 64
Stability and Reactivity
80:20 0 00 80:20 0 38 Formic acid 2 05
50:50 1 65 50:50 1 97 t-Butyl alcohol −3 2
20:80 3 05 10:90 3 28 90:10 acetone:water −1 85
0:100 3 49 90:10 dioxane:water −2 03
a. From A. H. Fainberg and S. Winstein, J. Am. Chem. Soc., 78, 2770 (1956).
Several other treatments of solvent effects on solvolysis rates have been
developed. 152 The equations typically include several terms related to: (a) macroscopic
nonspecific solvent properties, such as the dipole moment and dielectric constant;
(b) empirical polarity criteria, such as E (30); (c) solvent electrophilicity and nucle-
T
ophilicity parameters; and (d) terms related to solvent cohesivity. The last term accounts
for the difference in work required to disrupt structure within the solvent, when, for
example, there is expansion in volume between reactants and the TS.
3.8.2. Examples of Specific Solvent Effects
The electrostatic solvent effects discussed in the preceding paragraphs are not the
only possible modes of interaction of solvent with reactants and TS. Specific structural
effects may cause either the reactants or the TS to be particularly strongly solvated.
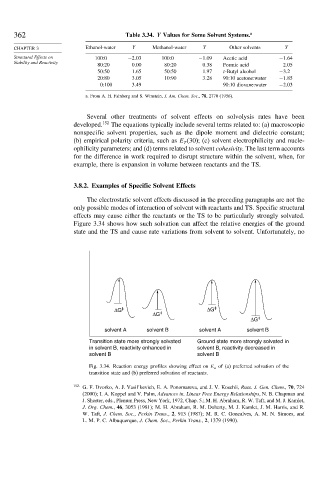
Figure 3.34 shows how such solvation can affect the relative energies of the ground
state and the TS and cause rate variations from solvent to solvent. Unfortunately, no
ΔG ‡ ΔG ‡
ΔG ‡
ΔG ‡
solvent A solvent B solvent A solvent B
Transition state more strongly solvated Ground state more strongly solvated in
in solvent B, reactivity enhanced in solvent B, reactivity decreased in
solvent B solvent B
Fig. 3.34. Reaction energy profiles showing effect on E a of (a) preferred solvation of the
transition state and (b) preferred solvation of reactants.
152
G. F. Dvorko, A. J. Vasil’kevich, E. A. Ponomareva, and J. V. Koschii, Russ. J. Gen. Chem., 70, 724
(2000); I. A. Koppel and V. Palm, Advances in. Linear Free Energy Relationships, N. B. Chapman and
J. Shorter, eds., Plenum Press, New York, 1972, Chap. 5.; M. H. Abraham, R. W. Taft, and M. J. Kamlet,
J. Org. Chem., 46, 3053 (1981); M. H. Abraham, R. M. Doherty, M. J. Kamlet, J. M. Harris, and R.
W. Taft, J. Chem. Soc., Perkin Trans., 2, 913 (1987); M. R. C. Goncalves, A. M. N. Simoes, and
L. M. P. C. Albuquerque, J. Chem. Soc., Perkin Trans., 2, 1379 (1990).