Page 383 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 383
364 When added to nonpolar solvents, the crown ethers increase the solubility of ionic
materials. For example, in the presence of 18-crown-6, potassium fluoride is soluble
CHAPTER 3 in benzene and acts as a reactive nucleophile:
Structural Effects on
Stability and Reactivity 18-crown-6
CH (CH ) Br + KF CH (CH ) F
3
2 7
3
2 7
benzene Ref. 154
In the absence of the polyether, potassium fluoride is insoluble in benzene and
unreactive toward alkyl halides. Similar enhancement of solubility and reactivity of
other salts is observed in the presence of crown ethers. The solubility and reactivity
enhancement results because the ionic compound is dissociated to a tightly complexed
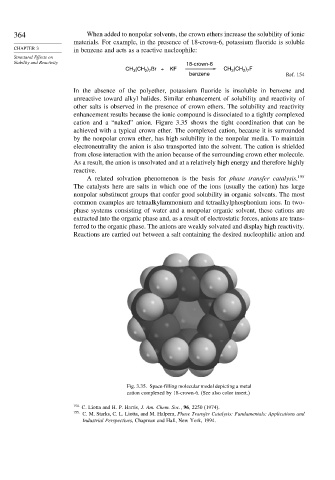
cation and a “naked” anion. Figure 3.35 shows the tight coordination that can be
achieved with a typical crown ether. The complexed cation, because it is surrounded
by the nonpolar crown ether, has high solubility in the nonpolar media. To maintain
electroneutrality the anion is also transported into the solvent. The cation is shielded
from close interaction with the anion because of the surrounding crown ether molecule.
As a result, the anion is unsolvated and at a relatively high energy and therefore highly
reactive.
A related solvation phenomenon is the basis for phase transfer catalysis. 155
The catalysts here are salts in which one of the ions (usually the cation) has large
nonpolar substituent groups that confer good solubility in organic solvents. The most
common examples are tetraalkylammonium and tetraalkylphosphonium ions. In two-
phase systems consisting of water and a nonpolar organic solvent, these cations are
extracted into the organic phase and, as a result of electrostatic forces, anions are trans-
ferred to the organic phase. The anions are weakly solvated and display high reactivity.
Reactions are carried out between a salt containing the desired nucleophilic anion and
Fig. 3.35. Space-filling molecular model depicting a metal
cation complexed by 18-crown-6. (See also color insert.)
154 C. Liotta and H. P. Harris, J. Am. Chem. Soc., 96, 2250 (1974).
155
C. M. Starks, C. L. Liotta, and M. Halpern, Phase Transfer Catalysis: Fundamentals: Applications and
Industrial Perspectives, Chapman and Hall, New York, 1994.