Page 387 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 387
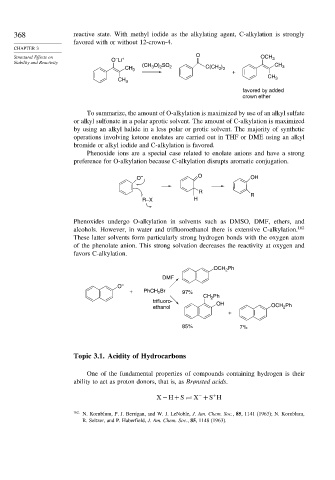
368 reactive state. With methyl iodide as the alkylating agent, C-alkylation is strongly
favored with or without 12-crown-4.
CHAPTER 3
O
Structural Effects on – + OCH 3
Stability and Reactivity O Li CH
)
CH 3 (CH 3 O) 2 SO 2 C(CH 3 3 3
+
CH
CH 3 3
favored by added
crown ether
To summarize, the amount of O-alkylation is maximized by use of an alkyl sulfate
or alkyl sulfonate in a polar aprotic solvent. The amount of C-alkylation is maximized
by using an alkyl halide in a less polar or protic solvent. The majority of synthetic
operations involving ketone enolates are carried out in THF or DME using an alkyl
bromide or alkyl iodide and C-alkylation is favored.
Phenoxide ions are a special case related to enolate anions and have a strong
preference for O-alkylation because C-alkylation disrupts aromatic conjugation.
O – O OH
R
R
R–X H
Phenoxides undergo O-alkylation in solvents such as DMSO, DMF, ethers, and
alcohols. However, in water and trifluoroethanol there is extensive C-alkylation. 162
These latter solvents form particularly strong hydrogen bonds with the oxygen atom
of the phenolate anion. This strong solvation decreases the reactivity at oxygen and
favors C-alkylation.
Ph
OCH 2
DMF
O –
+ PhCH Br 97%
2
CH Ph
2
trifluoro-
OH
ethanol OCH Ph
2
+
85% 7%
Topic 3.1. Acidity of Hydrocarbons
One of the fundamental properties of compounds containing hydrogen is their
ability to act as proton donors, that is, as Brønsted acids.
+
X −H+S X +S H
−
162
N. Kornblum, P. J. Berrigan, and W. J. LeNoble, J. Am. Chem. Soc., 85, 1141 (1963); N. Kornblum,
R. Seltzer, and P. Haberfield, J. Am. Chem. Soc., 85, 1148 (1963).