Page 363 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 363
+
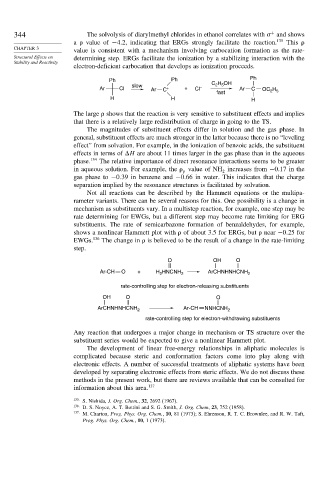
344 The solvolysis of diarylmethyl chlorides in ethanol correlates with and shows
a value of −4 2, indicating that ERGs strongly facilitate the reaction. 135 This
CHAPTER 3
value is consistent with a mechanism involving carbocation formation as the rate-
Structural Effects on determining step. ERGs facilitate the ionization by a stabilizing interaction with the
Stability and Reactivity
electron-deficient carbocation that develops as ionization proceeds.
Ph Ph C H OH Ph
slow 2 5
Ar Cl Ar C + + Cl – Ar C OC H
fast 2 5
H H H
The large shows that the reaction is very sensitive to substituent effects and implies
that there is a relatively large redistribution of charge in going to the TS.
The magnitudes of substituent effects differ in solution and the gas phase. In
general, substituent effects are much stronger in the latter because there is no “leveling
effect” from solvation. For example, in the ionization of benzoic acids, the substituent
effects in terms of H are about 11 times larger in the gas phase than in the aqueous
phase. 134 The relative importance of direct resonance interactions seems to be greater
in aqueous solution. For example, the value of NH increases from −0 17 in the
2
p
gas phase to −0 39 in benzene and −0 66 in water. This indicates that the charge
separation implied by the resonance structures is facilitated by solvation.
Not all reactions can be described by the Hammett equations or the multipa-
rameter variants. There can be several reasons for this. One possibility is a change in
mechanism as substituents vary. In a multistep reaction, for example, one step may be
rate determining for EWGs, but a different step may become rate limiting for ERG
substituents. The rate of semicarbazone formation of benzaldehydes, for example,
shows a nonlinear Hammett plot with of about 3.5 for ERGs, but near −0 25 for
EWGs. 136 The change in is believed to be the result of a change in the rate-limiting
step.
O OH O
Ar-CH O + H HNCNH 2 ArCHNHNHCNH 2
2
rate-controlling step for electron-releasing substituents
OH O O
ArCHNHNHCNH 2 Ar-CH NNHCNH 2
rate-controlling step for electron-withdrawing substituents
Any reaction that undergoes a major change in mechanism or TS structure over the
substituent series would be expected to give a nonlinear Hammett plot.
The development of linear free-energy relationships in aliphatic molecules is
complicated because steric and conformation factors come into play along with
electronic effects. A number of successful treatments of aliphatic systems have been
developed by separating electronic effects from steric effects. We do not discuss these
methods in the present work, but there are reviews available that can be consulted for
information about this area. 137
135 S. Nishida, J. Org. Chem., 32, 2692 (1967).
136 D. S. Noyce, A. T. Bottini and S. G. Smith, J. Org. Chem, 23, 752 (1958).
137
M. Charton, Prog. Phys. Org. Chem., 10, 81 (1973); S. Ehrenson, R. T. C. Brownlee, and R. W. Taft,
Prog. Phys. Org. Chem., 10, 1 (1973).