Page 341 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 341
322 anions. In a comparison of the acidity of acetic acid (pK 4.7) with that of alcohols (pK
16–18), the issue is the relative importance of the C=O bond dipole and resonance
CHAPTER 3
in stabilizing the carboxylate anion. These approaches have led to estimates ranging
Structural Effects on from 50 to 80% of the stabilization being polar in origin.
Stability and Reactivity
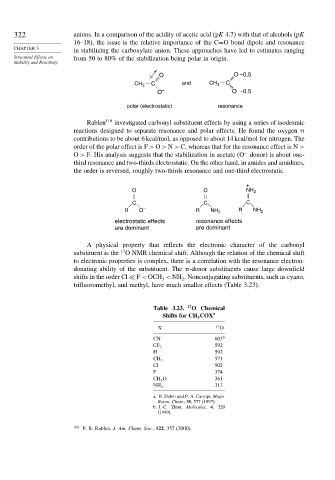
O --- O –0.5
CH 3 C and CH 3 C ---
O – O –0.5
polar (electrostatic) resonance
Rablen 110 investigated carbonyl substituent effects by using a series of isodesmic
reactions designed to separate resonance and polar effects. He found the oxygen
contributions to be about 6 kcal/mol, as opposed to about 14 kcal/mol for nitrogen. The
order of the polar effect is F > O > N > C, whereas that for the resonance effect is N >
O > F. His analysis suggests that the stabilization in acetate (O donor) is about one-
−
third resonance and two-thirds electrostatic. On the other hand, in amides and amidines,
the order is reversed, roughly two-thirds resonance and one-third electrostatic.
+
O O NH 2
C C C
R O – R NH 2 R NH 2
electrostatic effects resonance effects
are dominant are dominant
A physical property that reflects the electronic character of the carbonyl
17
substituent is the O NMR chemical shift. Although the relation of the chemical shift
to electronic properties is complex, there is a correlation with the resonance electron-
donating ability of the substituent. The -donor substituents cause large downfield
shifts in the order Cl
F < OCH < NH . Nonconjugating substituents, such as cyano,
3 2
trifluoromethyl, and methyl, have much smaller effects (Table 3.23).
Table 3.23. 17 O Chemical
Shifts for CH COX a
3
X 17 O
CN 603 b
592
CF 3
H 592
571
CH 3
Cl 502
F 374
CH 3 O 361
313
NH 2
a. H. Dahm and P. A. Carrupt, Magn.
Reson. Chem., 35, 577 (1997).
b. J.-C. Zhuo, Molecules, 4, 320
(1999).
110
P. R. Rablen, J. Am. Chem. Soc., 122, 357 (2000).