Page 335 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 335
316
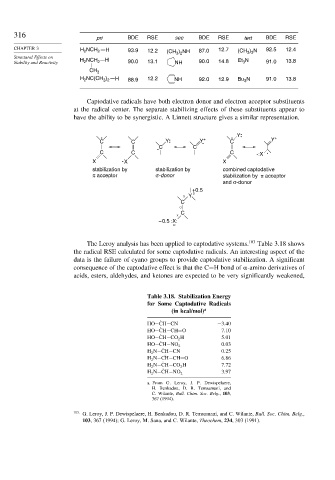
pri BDE RSE sec BDE RSE tert BDE RSE
CHAPTER 3 H NCH 2 H 93.9 12.2 ) NH 87.0 12.7 (CH 3 3 92.5 12.4
) N
2
Structural Effects on (CH 3 2
2
Stability and Reactivity H NCH 2 H 90.0 13.1 NH 90.0 14.8 Et 3 N 91.0 13.8
CH 3
NC(CH ) 12.2
H 2 3 2 H 88.9 NH 92.0 12.9 Bu 3 N 91.0 13.8
Captodative radicals have both electron donor and electron acceptor substituents
at the radical center. The separate stabilizing effects of these substituents appear to
have the ability to be synergistic. A Linnett structure gives a similar representation.
Y +
C C Y Y + C Y
C C
C C C -:X
X X X
stabilization by stabilization by combined captodative
π acceptor σ-donor stabilization by π acceptor
and σ-donor
+0.5
X Y O
C
O
C
X
– 0.5 :X:
O
The Leroy analysis has been applied to captodative systems. 103 Table 3.18 shows
the radical RSE calculated for some captodative radicals. An interesting aspect of the
data is the failure of cyano groups to provide captodative stabilization. A significant
consequence of the captodative effect is that the C−H bond of -amino derivatives of
acids, esters, aldehydes, and ketones are expected to be very significantly weakened,
Table 3.18. Stabilization Energy
for Some Captodative Radicals
(in kcal/mol) a
HO− ˙ CH−CN −3 40
HO− ˙ CH−CH=O 7 10
HO− ˙ CH−CO 2 H 5 01
0 03
HO− ˙ CH−NO 2
H 2 N− ˙ CH−CN 0 25
H 2 N− ˙ CH−CH=O 6 86
H 2 N− ˙ CH−CO 2 H 7 72
3 97
H 2 N− ˙ CH−NO 2
a. From G. Leroy, J. P. Dewispelaere,
H. Benkadou, D. R. Temsamani, and
C. Wilante, Bull. Chim. Soc. Belg., 103,
367 (1994).
103
G. Leroy, J. P. Dewispelaere, H. Benkadou, D. R. Temsamani, and C. Wilante, Bull. Soc. Chim. Belg.,
103, 367 (1994); G. Leroy, M. Sana, and C. Wilante, Theochem, 234, 303 (1991).