Page 331 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 331
312 A good place to begin discussion of substituent effects on radicals is by
considering the most common measure of radical stability. Radical stabilization is
CHAPTER 3 often defined by comparing C−H bond dissociation energies (BDE). For substituted
Structural Effects on methanes, the energy of the reaction should reflect any stabilizing features in the
Stability and Reactivity
radical X−CH .
2
. .
X −CH −H → X −CH +H
2 2
The BDEs are usually tabulated as the enthalpy change for the reaction at 298 K. The
values can be determined experimentally 92 or calculated theoretically. Table 3.2 B
(p. 258) gives some C−H BDE for important C−H bonds. The BDEs become smaller
in the order CH > pri > sec > tert. The relatively low enthalpies of the dissociation
3
for forming allyl and benzyl radicals by removal of hydrogen from propene and
toluene, respectively, is due to the stabilization of these radicals by delocalization.
2
On the other hand the C−H bond to sp (ethene, benzene) and sp (ethyne) carbon
3
are substantially stronger than bonds to sp carbon. The BDEs correlate with the ease
of formation of the corresponding radicals. 93 The reactivity of C−H groups toward
radicals that abstract hydrogen is pri < sec < tert. Vinyl and phenyl substituents at
a reaction site weaken the C−H bond and enhance reactivity. On the other hand,
2
hydrogen abstraction from an sp (vinyl or aryl) C−H bond or from a sp carbon in
a terminal alkyne is difficult because of the increased strength of the corresponding
C−H bonds.
The trend of reactivity tert > sec > pri is consistently observed in various
hydrogen atom abstraction reactions, but the range of reactivity is determined by the
nature of the reacting radical. The relative reactivity of pri, sec, and tert positions
toward hydrogen abstraction by methyl radicals is 1:4.8:61. 94 An allylic or benzylic
hydrogen is more reactive toward a methyl radical by a factor of about 9, compared
to an unsubstituted C−H. The relative reactivity toward the t-butoxy radical is pri:1,
sec: 10, tert: 50. 95 In the gas phase, the bromine atom is much more selective, with
relative reactivities of pri:1, sec: 250, tert: 6300. 96 Data for other types of radicals
have been obtained and tabulated. 96
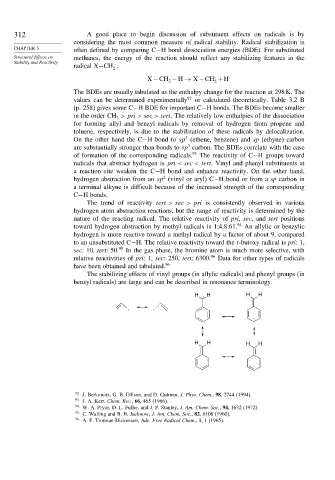
The stabilizing effects of vinyl groups (in allylic radicals) and phenyl groups (in
benzyl radicals) are large and can be described in resonance terminology.
H . H H H
. .
.
H H H H
. .
92 J. Berkowitz, G. B. Ellison, and D. Gutman, J. Phys. Chem., 98, 2744 (1994).
93
J. A. Kerr, Chem. Rev., 66, 465 (1966).
94 W. A. Pryor, D. L. Fuller, and J. P. Stanley, J. Am. Chem. Soc., 94, 1632 (1972).
95 C. Walling and B. B. Jacknow, J. Am. Chem. Soc., 82, 6108 (1960).
96
A. F. Trotman-Dickenson, Adv. Free Radical Chem., 1, 1 (1965).