Page 315 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 315
296 sense will also do so in a kinetic sense. Each of these approaches pertains to series of
reactions that proceed by similar mechanisms, because they are based upon a series
CHAPTER 3 of closely related reaction energy profiles. If substituents have a strong enough effect
Structural Effects on to change the mechanism, for example, a change in the rate-determining step, the
Stability and Reactivity
relationships cannot be expected to hold.
3.3.3. Curtin-Hammett Principle.
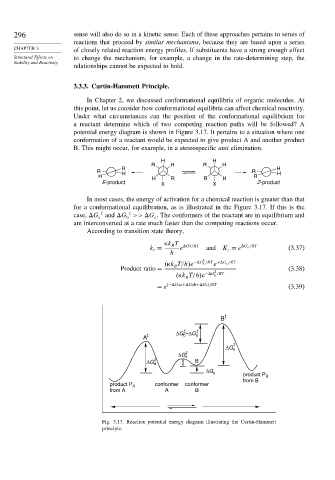
In Chapter 2, we discussed conformational equilibria of organic molecules. At
this point, let us consider how conformational equilibria can affect chemical reactivity.
Under what circumstances can the position of the conformational equilibrium for
a reactant determine which of two competing reaction paths will be followed? A
potential energy diagram is shown in Figure 3.17. It pertains to a situation where one
conformation of a reactant would be expected to give product A and another product
B. This might occur, for example, in a stereospecific anti elimination.
H H
R H R H
R H
R H R H
H H R R H R
E-product X X Z-product
In most cases, the energy of activation for a chemical reaction is greater than that
for a conformational equilibration, as is illustrated in the Figure 3.17. If this is the
‡
‡
case, G and G >> G . The conformers of the reactant are in equilibrium and
b
c
a
are interconverted at a rate much faster than the competing reactions occur.
According to transition state theory,
k T G‡/RT G c /RT
B
k = e and K = e (3.37)
c
r
h
‡
e
k T/h e − G a /RT + G c /RT
B
Product ratio = ‡ (3.38)
k T/h e − G /RT
b
B
= e − G‡a+ G‡b+ Gc /RT (3.39)
B
ΔG –ΔG a
b
A
ΔG b
ΔG c
ΔG a B
ΔG
c
product P B
from B
product P A conformer conformer
from A A B
Fig. 3.17. Reaction potential energy diagram illustrating the Curtin-Hammett
principle.