Page 314 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 314
With these two relationships, if reliable values of W and W can be determined 295
R P
˜
and G established for a reaction series, the activation barriers can be calculated
o
from the thermodynamic data that provide G . Relationships of this kind have been SECTION 3.3
shown to occur in many different organic reactions, so the principles appear to have General Relationships
between Thermodynamic
considerable generality. For example, Guthrie investigated both the addition (Step 2) Stability and Reaction
and elimination (Step 4) parts of the aldol condensation reaction (see p. 283–285). 62 Rates
Steps (1) and (3) are proton transfers that are fast under the reaction conditions.
O
O O – O O –
– OH R 2 C R 3
2 3
R 1 C CH 3 R 1 C CH 2 R 1 C CH CR R
2
(1) (2)
O O – O OH O
H O – OH
2
2 3
2 3
2 3
R 1 C CH 2 CR R R 1 C CH 2 CR R R 1 C CH CR R
(3) (4)
A series of acceptors CH =O CH CH=O PhCH=O CH C=O and a series of
3
2
3 2
−
−
enolates ( CH CH=O CH COCH CH COPh were examined and good correla-
−
2
2
3
2
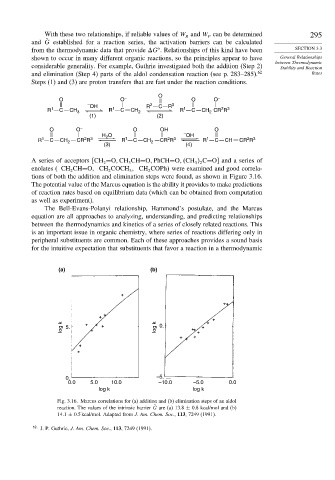
tions of both the addition and elimination steps were found, as shown in Figure 3.16.
The potential value of the Marcus equation is the ability it provides to make predictions
of reaction rates based on equilibrium data (which can be obtained from computation
as well as experiment).
The Bell-Evans-Polanyi relationship, Hammond’s postulate, and the Marcus
equation are all approaches to analyzing, understanding, and predicting relationships
between the thermodynamics and kinetics of a series of closely related reactions. This
is an important issue in organic chemistry, where series of reactions differing only in
peripheral substituents are common. Each of these approaches provides a sound basis
for the intuitive expectation that substituents that favor a reaction in a thermodynamic
(a) (b)
log k 5. log k 0.
0. –5.
0.0 5.0 10.0 –10.0 –5.0 0.0
log k log k
Fig. 3.16. Marcus correlations for (a) addition and (b) elimination steps of an aldol
reaction. The values of the intrinsic barrier ˜ G are (a) 13.8 ± 0.8 kcal/mol and (b)
14.1 ± 0.5 kcal/mol. Adapted from J. Am. Chem. Soc., 113, 7249 (1991).
62
J. P. Guthrie, J. Am. Chem. Soc., 113, 7249 (1991).