Page 306 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 306
Thus, whenever competing or successive reaction products can come to 287
equilibrium, the product composition will reflect relative stability and be subject to
thermodynamic control. If product composition is governed by competing rates, the SECTION 3.3
reaction is under kinetic control. A given reaction may be subject to either thermody- General Relationships
between Thermodynamic
namic or kinetic control, depending on the conditions. Stability and Reaction
The idea of kinetic versus thermodynamic control can be illustrated by a brief Rates
discussion of the formation of enolate anions from unsymmetrical ketones. This is a
very important matter for synthesis and is discussed more fully in Chapter 6 and in
Section 1.1.2 in Part B. Most ketones can give rise to more than one enolate. Many
studies have shown that the ratio among the possible enolates that are formed depends
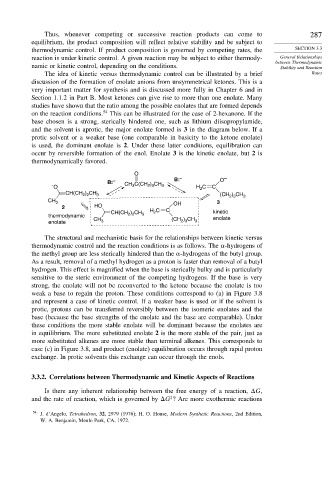
on the reaction conditions. 54 This can be illustrated for the case of 2-hexanone. If the
base chosen is a strong, sterically hindered one, such as lithium diisopropylamide,
and the solvent is aprotic, the major enolate formed is 3 in the diagram below. If a
protic solvent or a weaker base (one comparable in basicity to the ketone enolate)
is used, the dominant enolate is 2. Under these latter conditions, equilibration can
occur by reversible formation of the enol. Enolate 3 is the kinetic enolate, but 2 is
thermodynamically favored.
O
B: – B: – O –
– O CH 3 C(CH 2 ) 3 CH 3 H 2 C C
CH(CH ) CH 3 (CH 2 3 3
) CH
2 2
CH 3 OH 3
2 HO
CH(CH ) CH H 2 C C kinetic
thermodynamic 2 2 3
) CH
CH (CH 2 3 enolate
enolate 3 3
The structural and mechanistic basis for the relationships between kinetic versus
thermodynamic control and the reaction conditions is as follows. The -hydrogens of
the methyl group are less sterically hindered than the -hydrogens of the butyl group.
As a result, removal of a methyl hydrogen as a proton is faster than removal of a butyl
hydrogen. This effect is magnified when the base is sterically bulky and is particularly
sensitive to the steric environment of the competing hydrogens. If the base is very
strong, the enolate will not be reconverted to the ketone because the enolate is too
weak a base to regain the proton. These conditions correspond to (a) in Figure 3.8
and represent a case of kinetic control. If a weaker base is used or if the solvent is
protic, protons can be transferred reversibly between the isomeric enolates and the
base (because the base strengths of the enolate and the base are comparable). Under
these conditions the more stable enolate will be dominant because the enolates are
in equilibrium. The more substituted enolate 2 is the more stable of the pair, just as
more substituted alkenes are more stable than terminal alkenes. This corresponds to
case (c) in Figure 3.8, and product (enolate) equilibration occurs through rapid proton
exchange. In protic solvents this exchange can occur through the enols.
3.3.2. Correlations between Thermodynamic and Kinetic Aspects of Reactions
Is there any inherent relationship between the free energy of a reaction, G,
‡
and the rate of reaction, which is governed by G ? Are more exothermic reactions
54
J. d’Angelo, Tetrahedron, 32, 2979 (1976); H. O. House, Modern Synthetic Reactions, 2nd Edition,
W. A. Benjamin, Menlo Park, CA, 1972.