Page 890 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 890
874 +
b + –
a c – b b
CHAPTER 10 a c or a b c a c
Concerted Pericyclic d e d e d e d e
Reactions
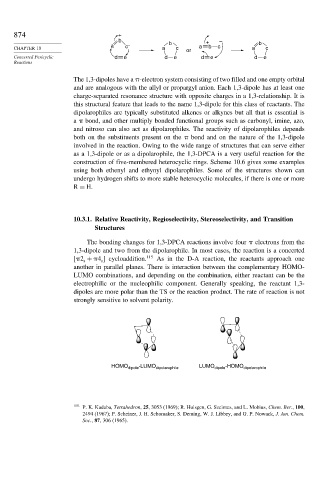
The 1,3-dipoles have a -electron system consisting of two filled and one empty orbital
and are analogous with the allyl or propargyl anion. Each 1,3-dipole has at least one
charge-separated resonance structure with opposite charges in a 1,3-relationship. It is
this structural feature that leads to the name 1,3-dipole for this class of reactants. The
dipolarophiles are typically substituted alkenes or alkynes but all that is essential is
a bond, and other multiply bonded functional groups such as carbonyl, imine, azo,
and nitroso can also act as dipolarophiles. The reactivity of dipolarophiles depends
both on the substituents present on the bond and on the nature of the 1,3-dipole
involved in the reaction. Owing to the wide range of structures that can serve either
as a 1,3-dipole or as a dipolarophile, the 1,3-DPCA is a very useful reaction for the
construction of five-membered heterocyclic rings. Scheme 10.6 gives some examples
using both ethenyl and ethynyl dipolarophiles. Some of the structures shown can
undergo hydrogen shifts to more stable heterocyclic molecules, if there is one or more
R = H.
10.3.1. Relative Reactivity, Regioselectivity, Stereoselectivity, and Transition
Structures
The bonding changes for 1,3-DPCA reactions involve four electrons from the
1,3-dipole and two from the dipolarophile. In most cases, the reaction is a concerted
[ 2 + 4 ] cycloaddition. 115 As in the D-A reaction, the reactants approach one
s
s
another in parallel planes. There is interaction between the complementary HOMO-
LUMO combinations, and depending on the combination, either reactant can be the
electrophilic or the nucleophilic component. Generally speaking, the reactant 1,3-
dipoles are more polar than the TS or the reaction product. The rate of reaction is not
strongly sensitive to solvent polarity.
HOMO dipole -LUMO dipolarophile LUMO dipole -HOMO dipolarophile
115
P. K. Kadaba, Tetrahedron, 25, 3053 (1969); R. Huisgen, G. Szeimes, and L. Mobius, Chem. Ber., 100,
2494 (1967); P. Scheiner, J. H. Schomaker, S. Deming, W. J. Libbey, and G. P. Nowack, J. Am. Chem.
Soc., 87, 306 (1965).