Page 894 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 894
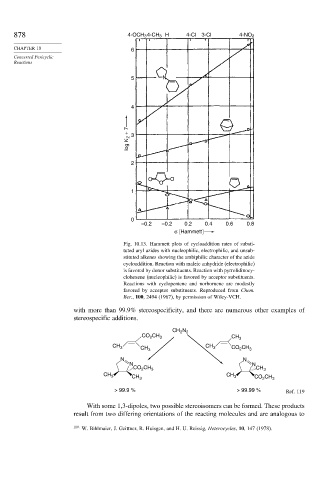
878 4-OCH 34-CH 3 H 4-Cl 3-Cl 4-NO 2
CHAPTER 10 6
Concerted Pericyclic
Reactions
5 N
4
log K Z + 7 3
2
O O
O
1
0
–0.2 –0.2 0.2 0.4 0.6 0.8
σ [Hammett]
Fig. 10.13. Hammett plots of cycloaddition rates of substi-
tuted aryl azides with nucleophilic, electrophilic, and unsub-
stituted alkenes showing the ambiphilic character of the azide
cycloaddition. Reaction with maleic anhydride (electrophilic)
is favored by donor substituents. Reaction with pyrrolidinocy-
clohexene (nucleophilic) is favored by acceptor substituents.
Reactions with cyclopentene and norbornene are modestly
favored by acceptor substituents. Reproduced from Chem.
Ber., 100, 2494 (1967), by permission of Wiley-VCH.
with more than 99.9% stereospecificity, and there are numerous other examples of
stereospecific additions.
CH N
2 2
CO CH 3 CH 3
2
CH 3 CH 3 CO CH
CH 3 2 3
N N
N N
CO CH 3 CH 3
2
CH 3 CH 3
CH 3 CO CH 3
2
> 99.9 % > 99.99 % Ref. 119
With some 1,3-dipoles, two possible stereoisomers can be formed. These products
result from two differing orientations of the reacting molecules and are analogous to
119
W. Bihlmaier, J. Geittner, R. Huisgen, and H. U. Reissig, Heterocycles, 10, 147 (1978).