Page 892 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 892
876 There have been many computational analyses of 1,3-DPCA TSs, and they are
generally regarded to be aromatic in character. Typical TSs are characterized by
CHAPTER 10 aromatic NICS values. 116 The ring current associated with this aromaticity is primarily
Concerted Pericyclic due to the six electrons undergoing bonding changes. 117 The orbital interactions in the
Reactions
cyclic TS serve as the focal point for discussion of relative reactivity, regioselectivity,
and stereoselectivity of 1,3-DPCA reactions.
The most widely applied interpretation of substituent effects on relative reactivity
is based on FMO theory. According to FMO theory, interacting orbitals are most
stabilized when they are closest in energy. Substituent effects on dipolar cycloadditions
can be interpreted in terms of matching of HOMO and LUMO orbitals of the two
reactants. 118 This is the same concept used in applying FMO theory to D-A reactions
(see p. 844–848). In the D-A reaction, it is fairly clear which reactant is electrophilic
and which is nucleophilic, and the interpretation of substituent effects follows directly.
This choice is not always so obvious for 1,3-DPCA reactions. In fact, for several of
the 1,3-dipoles both EWGs and ERGs in the dipolarophile enhance reactivity. These
1,3-dipoles are called ambiphilic. Let us look carefully to see why they have this
property.
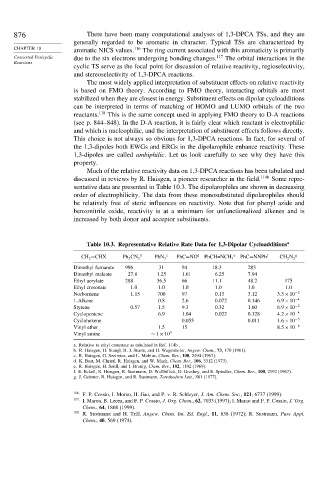
Much of the relative reactivity data on 1,3-DPCA reactions has been tabulated and
discussed in reviews by R. Huisgen, a pioneer researcher in the field. 114b Some repre-
sentative data are presented in Table 10.3. The dipolarophiles are shown in decreasing
order of electrophilicity. The data from these monosubstituted dipolarophiles should
be relatively free of steric influences on reactivity. Note that for phenyl azide and
benzonitrile oxide, reactivity is at a minimum for unfunctionalized alkenes and is
increased by both donor and acceptor substituents.
Table 10.3. Representative Relative Rate Data for 1,3-Dipolar Cycloadditions a
CH 2 =CHX Ph 2 CN 2 b PhN 3 c PhC≡NO d PhCH=NCH 3 e PhC≡NNPh f CH 2 N 2 g
Dimethyl fumarate 996 31 94 18.3 283
Dimethyl maleate 27 8 1.25 1.61 6.25 7.94
Ethyl acrylate 288 36.5 66 11.1 48.2 175
Ethyl crotonate 1 0 1.0 1.0 1.0 1.0 1.0
Norbornene 1 15 700 97 0.13 3.12 3 3×10 −2
1-Alkene 0.8 2.6 0.072 0.146 6 9×10 −4
Styrene 0 57 1.5 9.3 0.32 1.60 6 9×10 −2
Cyclopentene 6.9 1.04 0.022 0.128 4 2×10 −4
Cyclohexene 0.055 0.011 1 6×10 −5
Vinyl ether 1.5 15 8 5×10 −6
Vinyl amine ∼ 1×10 5
a. Relative to ethyl crotonate as tabulated in Ref. 114b .
b. R. Huisgen, H. Stangl, H. J. Sturm, and H. Wagenhofer, Angew. Chem., 73, 170 (1961).
c. R. Huisgen, G. Szeimies, and L. Mobius, Chem. Ber., 100, 2494 (1967).
d. K. Bast, M. Christl, R. Huisgen, and W. Mack, Chem. Ber., 106, 3312 (1973).
e. R. Huisgen, H. Seidl, and I. Brunig, Chem. Ber., 102, 1102 (1969).
f. E. Eckell, R. Huisgen, R. Sustmann, D. Wallbillich, D. Grashey, and E. Spindler, Chem. Ber., 100, 2192 (1967).
g. J. Geittner, R. Huisgen, and R. Sustmann, Tetrahedron Lett., 881 (1977).
116
F. P. Cossio, I. Morao, H. Jiao, and P. v. R. Schleyer, J. Am. Chem. Soc., 121, 6737 (1999).
117 I. Maroa, B. Lecea, and F. P. Cossio, J. Org. Chem., 62, 7033 (1997); I. Marao and F. P. Cossio, J.‘Org.
Chem., 64, 1868 (1999).
118
R. Sustmann and H. Trill, Angew. Chem. Int. Ed. Engl., 11, 838 (1972); R. Sustmann, Pure Appl.
Chem., 40, 569 (1974).