Page 893 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 893
In addition to the electronic effects of substituents, several other structural features 877
affect the reactivity of dipolarophiles. Strain increases reactivity. Norbornene, for
example, is consistently more reactive than cyclopentene in 1,3-dipolar cycloadditions. SECTION 10.3
Cyclopentene is also more reactive than cyclohexene. Conjugating substituents, such 1,3-Dipolar
Cycloaddition Reactions
as the phenyl group in styrene, usually increase reactivity of dipolarophiles (compare
styrene with 1-alkenes in Table 10.3).
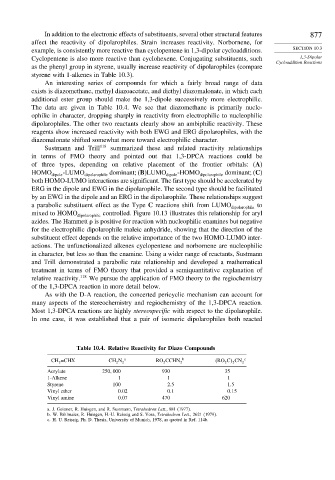
An interesting series of compounds for which a fairly broad range of data
exists is diazomethane, methyl diazoacetate, and diethyl diazomalonate, in which each
additional ester group should make the 1,3-dipole successively more electrophilic.
The data are given in Table 10.4. We see that diazomethane is primarily nucle-
ophilic in character, dropping sharply in reactivity from electrophilic to nucleophilic
dipolarophiles. The other two reactants clearly show an ambiphilic reactivity. These
reagents show increased reactivity with both EWG and ERG dipolarophiles, with the
diazomalonate shifted somewhat more toward electrophilic character.
Sustmann and Trill 118 summarized these and related reactivity relationships
in terms of FMO theory and pointed out that 1,3-DPCA reactions could be
of three types, depending on relative placement of the frontier orbitals: (A)
HOMO dipole -LUMO dipolarophile dominant; (B)LUMO dipole -HOMO dipolarophile dominant; (C)
both HOMO-LUMO interactions are significant. The first type should be accelerated by
ERG in the dipole and EWG in the dipolarophile. The second type should be facilitated
by an EWG in the dipole and an ERG in the dipolarophile. These relationships suggest
a parabolic substituent effect as the Type C reactions shift from LUMO dipolarophile to
mixed to HOMO dipolarophile controlled. Figure 10.13 illustrates this relationship for aryl
azides. The Hammett is positive for reaction with nucleophilic enamines but negative
for the electrophilic dipolarophile maleic anhydride, showing that the direction of the
substituent effect depends on the relative importance of the two HOMO-LUMO inter-
actions. The unfunctionalized alkenes cyclopentene and norbornene are nucleophilic
in character, but less so than the enamine. Using a wider range of reactants, Sustmann
and Trill demonstrated a parabolic rate relationship and developed a mathematical
treatment in terms of FMO theory that provided a semiquantitative explanation of
relative reactivity. 118 We pursue the application of FMO theory to the regiochemistry
of the 1,3-DPCA reaction in more detail below.
As with the D-A reaction, the concerted pericyclic mechanism can account for
many aspects of the stereochemistry and regiochemistry of the 1,3-DPCA reaction.
Most 1,3-DPCA reactions are highly stereospecific with respect to the dipolarophile.
In one case, it was established that a pair of isomeric dipolarophiles both reacted
Table 10.4. Relative Reactivity for Diazo Compounds
CH 2 =CHX CH 2 N 2 a RO 2 CCHN 2 b RO 2 C 2 CN 2 c
Acrylate 250 000 930 35
1-Alkene 1 1 1
Styrene 100 2 5 1 5
Vinyl ether 0 02 0 1 0 15
Vinyl amine 0 07 470 620
a. J. Geittner, R. Huisgen, and R. Sustmann, Tetrahedron Lett., 881 (1977).
b. W. Bihlmaier, R. Huisgen, H.-U. Reissig and S. Voss, Tetrahedron Lett., 2621 (1979).
c. H. U. Reissig, Ph. D. Thesis, University of Munich, 1978, as quoted in Ref. 114b.