Page 898 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 898
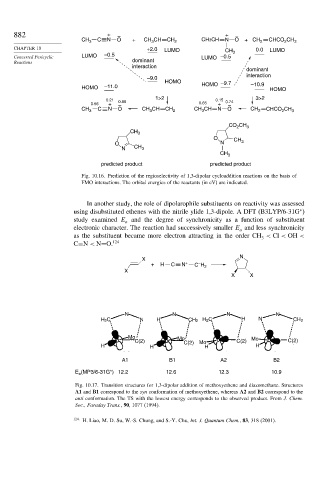
882 + – + –
CH 3 C N O + CH 3 CH CH 2 CH3CH N O + CH CHCO CH 3
2
2
CHAPTER 10 +2.0 LUMO CH 3 0.0 LUMO
Concerted Pericyclic LUMO –0.5 LUMO –0.5
Reactions dominant
interaction
dominant
interaction
–9.0
HOMO HOMO –9.7 –10.9
HOMO –11.0 HOMO
1>2 3>2
0.21 0.80 0.15 0.74
0.56 + – 0.65 + –
CH 3 C N O CH 3 CH CH 2 CH 3 CH N O CH 2 CHCO CH 3
2
CO CH 3
2
CH 3
O CH
O N 3
N CH 3
CH 3
predicted product predicted product
Fig. 10.16. Prediction of the regioselectivity of 1,3-dipolar cycloaddition reactions on the basis of
FMO interactions. The orbital energies of the reactants (in eV) are indicated.
In another study, the role of dipolarophile substituents on reactivity was assessed
∗
using disubstituted ethenes with the nitrile ylide 1,3-dipole. A DFT (B3LYP/6-31G )
study examined E and the degree of synchronicity as a function of substituent
a
electronic character. The reaction had successively smaller E and less synchronicity
a
as the substituent became more electron attracting in the order CH < Cl < OH <
3
C≡N < N=O. 124
N
X
–
+ H C N + C H 2
X
X X
N N N N
H 2C N H CH 2 H 2C H N CH 2
Mo Mo Mo
C(2) C(2) Mo C(2) C(2)
H H H H
A1 B1 A2 B2
E (MP3/6-31G*) 12.2 12.6 12.3 10.9
a
Fig. 10.17. Transition structures for 1,3-dipolar addition of methoxyethene and diazomethane. Structures
A1 and B1 correspond to the syn conformation of methoxyethene, whereas A2 and B2 correspond to the
anti conformation. The TS with the lowest energy corresponds to the observed product. From J. Chem.
Soc., Faraday Trans., 90, 1077 (1994).
124
H. Liao, M. D. Su, W.-S. Chung, and S.-Y. Chu, Int. J. Quantum Chem., 83, 318 (2001).