Page 902 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 902
886 rate-determining step in the reaction. Note that the ring opening is a stereospecific
electrocyclic process. (The stereochemistry of electrocyclic ring opening is discussed
CHAPTER 10
in Section 10.5).
Concerted Pericyclic
Reactions
10.3.3. Catalysis of 1,3-Dipolar Cycloaddition Reactions
The role of catalysts in 1,3-DPCA reactions is similar to that in D-A reactions.
Most catalysts are Lewis acids. Effective catalysts include Yb O SCF with
3 3
3
BINOL, 131 Mg -bis-oxazolines, 132 and oxazaborolidines. 133 Intramolecular nitrone
2+
cycloadditions can be facilitated by Lewis acids such as ZnCl . 134 The catalysts function
2
by enhancing the reactivity of the more electrophilic component of the reaction.
Although the diene is often nonpolar and inert to Lewis acids in D-A reactions, that is
not the case for 1,3-DPCA. Consideration of catalysts must include the potential inter-
action with both the dipole and dipolarophile. Catalyst interaction with the 1,3-dipole
is likely to be detrimental if the dipole is the more nucleophilic component of the
reaction. For example, with nitrones and enones, formation of a Lewis acid adduct with
the nitrone in competition with the enone is detrimental. One approach to this problem
is to use highly substituted catalysts that are selective for the less substituted reactant.
Bulky aryloxyaluminum compounds are excellent catalysts for nitrone cycloaddition
and also enhance regioselectivity. 135 The reaction of diphenylnitrone with enones is
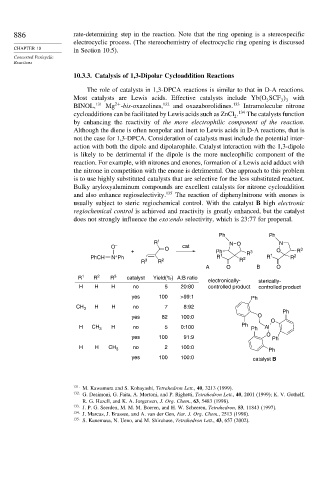
usually subject to steric regiochemical control. With the catalyst B high electronic
regiochemical control is achieved and reactivity is greatly enhanced, but the catalyst
does not strongly influence the exo:endo selectivity, which is 23:77 for propenal.
Ph Ph
R 1 NO N
O – O cat
+ Ph R 3 O R 3
+
PhCH N Ph 2 R 1 2 R 1 R 2
R 3 R R
A O B O
R 1 R 2 R 3 catalyst Yield(%) A:B ratio
electronically- sterically-
H H H no 5 20:80 controlled product controlled product
yes 100 >99:1 Ph
CH 3 H H no 7 8:92
Ph
yes 82 100:0 O
O
H CH 3 H no 5 0:100 Ph Ph Al
O
yes 100 91:9 Ph
H H no 2 100:0
CH 3
Ph
yes 100 100:0 catalyst B
131 M. Kawamura and S. Kobayashi, Tetrahedron Lett., 40, 3213 (1999).
132
G. Desimoni, G. Faita, A. Mortoni, and P. Righetti, Tetrahedron Lett., 40, 2001 (1999); K. V. Gothelf,
R. G. Hazell, and K. A. Jorgensen, J. Org. Chem., 63, 5483 (1998).
133 J. P. G. Seerden, M. M. M. Boeren, and H. W. Scheeren, Tetrahedron, 53, 11843 (1997).
134 J. Marcus, J. Brussee, and A. van der Gen, Eur. J. Org. Chem., 2513 (1998).
135
S. Kanemasa, N. Ueno, and M. Shirahase, Tetrahedron Lett., 43, 657 (2002).