Page 905 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 905
889
O SECTION 10.4
2+2 Cycloaddition
Reactions
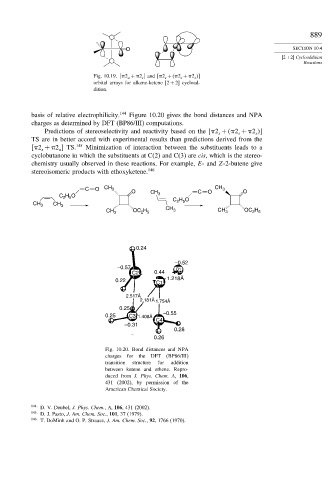
Fig. 10.19. [ 2 a + 2 s ] and [ 2 s + 2 s + 2 s ]
orbital arrays for alkene-ketene 2 + 2 cycload-
dition.
basis of relative electrophilicity. 144 Figure 10.20 gives the bond distances and NPA
charges as determined by DFT (BP86/III) computations.
Predictions of stereoselectivity and reactivity based on the [ 2 + 2 + 2 ]
s
s
s
TS are in better accord with experimental results than predictions derived from the
[ 2 + 2 ] TS. 145 Minimization of interaction between the substituents leads to a
s
a
cyclobutanone in which the substituents at C(2) and C(3) are cis, which is the stereo-
chemistry usually observed in these reactions. For example, E- and Z-2-butene give
stereoisomeric products with ethoxyketene. 146
C O CH 3 O C O CH 3 O
C H O CH 3
2 5
2 5
CH 3 CH 3 C H O
CH 3 OC H CH 3 CH 3 OC H
2 5
2 5
0.24
–0.52
–0.57 01
C2 0.44
0.22 1.218Å
C1
2.517Å
2.181Å 1.754Å
0.25
–0.55
0.25 C3 1.408Å
C4
–0.31
0.28
0.26
Fig. 10.20. Bond distances and NPA
charges for the DFT (BP86/III)
transition structure for addition
between ketene and ethene. Repro-
duced from J. Phys. Chem. A, 106,
431 (2002), by permission of the
American Chemical Society.
144 D. V. Deubel, J. Phys. Chem., A, 106, 431 (2002).
145 D. J. Pasto, J. Am. Chem. Soc., 101, 37 (1979).
146
T. DoMinh and O. P. Strausz, J. Am. Chem. Soc., 92, 1766 (1970).