Page 903 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 903
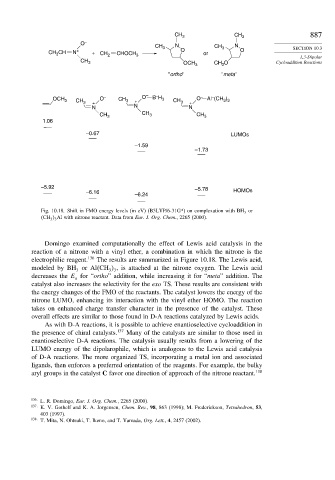
CH 3 CH 3 887
O –
CH 3 N CH 3 N SECTION 10.3
CH CH N + + CH 2 CHOCH 3 O or O
3
1,3-Dipolar
CH 3 OCH 3 CH 3 O Cycloaddition Reactions
"ortho" "meta"
–
–
OCH 3 CH 3 + O – CH 3 + O – B H 3 CH 3 + O – Al (CH )
3 3
N N N
CH 3 CH 3 CH 3
1.06
–0.67 LUMOs
–1.59
–1.73
–5.92 –5.78
–6.16 HOMOs
–6.24
Fig. 10.18. Shift in FMO energy levels (in eV) (B3LYP/6-31G*) on complexation with BH 3 or
CH 3 3 Al with nitrone reactant. Data from Eur. J. Org. Chem., 2265 (2000).
Domingo examined computationally the effect of Lewis acid catalysis in the
reaction of a nitrone with a vinyl ether, a combination in which the nitrone is the
electrophilic reagent. 136 The results are summarized in Figure 10.18. The Lewis acid,
modeled by BH or Al CH , is attached at the nitrone oxygen. The Lewis acid
3
3 3
decreases the E for “ortho” addition, while increasing it for “meta” addition. The
a
catalyst also increases the selectivity for the exo TS. These results are consistent with
the energy changes of the FMO of the reactants. The catalyst lowers the energy of the
nitrone LUMO, enhancing its interaction with the vinyl ether HOMO. The reaction
takes on enhanced charge transfer character in the presence of the catalyst. These
overall effects are similar to those found in D-A reactions catalyzed by Lewis acids.
As with D-A reactions, it is possible to achieve enantioselective cycloaddition in
the presence of chiral catalysts. 137 Many of the catalysts are similar to those used in
enantioselective D-A reactions. The catalysis usually results from a lowering of the
LUMO energy of the dipolarophile, which is analogous to the Lewis acid catalysis
of D-A reactions. The more organized TS, incorporating a metal ion and associated
ligands, then enforces a preferred orientation of the reagents. For example, the bulky
aryl groups in the catalyst C favor one direction of approach of the nitrone reactant. 138
136
L. R. Domingo, Eur. J. Org. Chem., 2265 (2000).
137 K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 98, 863 (1998); M. Frederickson, Tetrahedron, 53,
403 (1997).
138
T. Mita, N. Ohtsuki, T. Ikeno, and T. Yamada, Org. Lett., 4, 2457 (2002).