Page 908 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 908
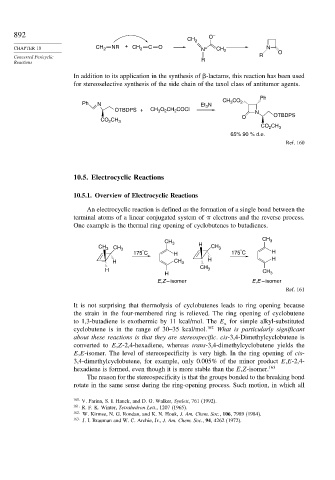
892 O –
CH 2
CHAPTER 10 CH 2 NR + CH 2 C O N + CH 2 N O
Concerted Pericyclic R
Reactions R
In addition to its application in the synthesis of -lactams, this reaction has been used
for stereoselective synthesis of the side chain of the taxol class of antitumor agents.
Ph
CO
CH 3
Ph N Et N 2
3
OTBDPS + CH O CH COCl N
2
3
2
O OTBDPS
CH
CO 2 3
CO CH 3
2
65% 90 % d.e.
Ref. 160
10.5. Electrocyclic Reactions
10.5.1. Overview of Electrocyclic Reactions
An electrocyclic reaction is defined as the formation of a single bond between the
terminal atoms of a linear conjugated system of electrons and the reverse process.
One example is the thermal ring opening of cyclobutenes to butadienes.
CH
CH 3 3
CH 3 CH 3 H CH 3
°
°
175 C H 175 C H
H CH 3 H H
H CH 3 CH
H 3
E,Z – isomer E,E – isomer
Ref. 161
It is not surprising that thermolysis of cyclobutenes leads to ring opening because
the strain in the four-membered ring is relieved. The ring opening of cyclobutene
to 1,3-butadiene is exothermic by 11 kcal/mol. The E for simple alkyl-substituted
a
cyclobutene is in the range of 30–35 kcal/mol. 162 What is particularly significant
about these reactions is that they are stereospecific. cis-3,4-Dimethylcyclobutene is
converted to E,Z-2,4-hexadiene, whereas trans-3,4-dimethylcyclobutene yields the
E,E-isomer. The level of stereospecificity is very high. In the ring opening of cis-
3,4-dimethylcyclobutene, for example, only 0.005% of the minor product E,E-2,4-
hexadiene is formed, even though it is more stable than the E,Z-isomer. 163
The reason for the stereospecificity is that the groups bonded to the breaking bond
rotate in the same sense during the ring-opening process. Such motion, in which all
160
V. Farina, S. I. Hauck, and D. G. Walker, Synlett, 761 (1992).
161 R. F. K. Winter, Tetrahedron Lett., 1207 (1965).
162 W. Kirmse, N. G. Rondan, and K. N. Houk, J. Am. Chem. Soc., 106, 7989 (1984).
163
J. I. Brauman and W. C. Archie, Jr., J. Am. Chem. Soc., 94, 4262 (1972).