Page 910 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 910
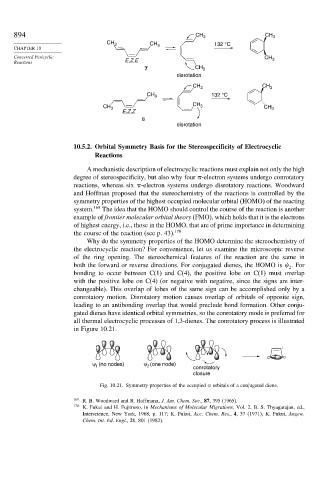
894 CH 3 CH 3
CH 3 CH 132 °C
CHAPTER 10 3
Concerted Pericyclic CH 3
Reactions E,Z,E
7 CH 3
disrotation
CH 3 CH 3
CH 3 132 °C
CH
CH 3 3 CH 3
E,Z,Z
8
disrotation
10.5.2. Orbital Symmetry Basis for the Stereospecificity of Electrocyclic
Reactions
A mechanistic description of electrocyclic reactions must explain not only the high
degree of stereospecificity, but also why four -electron systems undergo conrotatory
reactions, whereas six -electron systems undergo disrotatory reactions. Woodward
and Hoffman proposed that the stereochemistry of the reactions is controlled by the
symmetry properties of the highest occupied molecular orbital (HOMO) of the reacting
system. 169 The idea that the HOMO should control the course of the reaction is another
example of frontier molecular orbital theory (FMO), which holds that it is the electrons
of highest energy, i.e., those in the HOMO, that are of prime importance in determining
the course of the reaction (see p. 43). 170
Why do the symmetry properties of the HOMO determine the stereochemistry of
the electrocyclic reaction? For convenience, let us examine the microscopic reverse
of the ring opening. The stereochemical features of the reaction are the same in
both the forward or reverse directions. For conjugated dienes, the HOMO is
. For
2
bonding to occur between C(1) and C(4), the positive lobe on C(1) must overlap
with the positive lobe on C(4) (or negative with negative, since the signs are inter-
changeable). This overlap of lobes of the same sign can be accomplished only by a
conrotatory motion. Disrotatory motion causes overlap of orbitals of opposite sign,
leading to an antibonding overlap that would preclude bond formation. Other conju-
gated dienes have identical orbital symmetries, so the conrotatory mode is preferred for
all thermal electrocyclic processes of 1,3-dienes. The conrotatory process is illustrated
in Figure 10.21.
ψ (no nodes) ψ (one node) conrotatory
2
1
closure
Fig. 10.21. Symmetry properties of the occupied orbitals of a conjugated diene.
169 R. B. Woodward and R. Hoffmann, J. Am. Chem. Soc., 87, 395 (1965).
170
K. Fukui and H. Fujimoto, in Mechanisms of Molecular Migrations, Vol. 2, B. S. Thyagarajan, ed.,
Interscience, New York, 1968, p. 117; K. Fukui, Acc. Chem. Res., 4, 57 (1971); K. Fukui, Angew.
Chem. Int. Ed. Engl., 21, 801 (1982).