Page 907 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 907
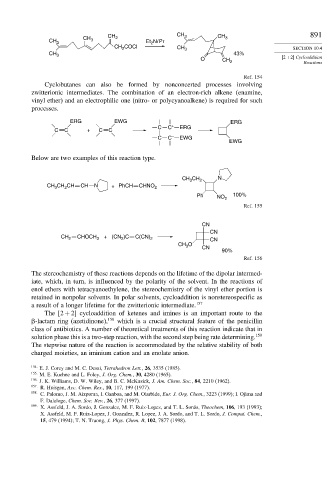
CH CH 2 CH 3 CH 3 891
CH 3 3 Et 2 Ni Pr
CH COCl CH 3 SECTION 10.4
2
CH 3 43%
O 2+2 Cycloaddition
CH 2
Reactions
Ref. 154
Cyclobutanes can also be formed by nonconcerted processes involving
zwitterionic intermediates. The combination of an electron-rich alkene (enamine,
vinyl ether) and an electrophilic one (nitro- or polycyanoalkene) is required for such
processes.
ERG EWG ERG
C C + ERG
C C + C C
C C – EWG
EWG
Below are two examples of this reaction type.
CH N
CH 3 2
CH CH CH CH N + PhCH CHNO 2
2
3
Ph NO 2 100%
Ref. 155
CN
CN
CH 2 CHOCH 3 + (CN )C C(CN) 2 CN
2
O
CH 3
CN
90%
Ref. 156
The stereochemistry of these reactions depends on the lifetime of the dipolar intermed-
iate, which, in turn, is influenced by the polarity of the solvent. In the reactions of
enol ethers with tetracyanoethylene, the stereochemistry of the vinyl ether portion is
retained in nonpolar solvents. In polar solvents, cycloaddition is nonstereospecific as
a result of a longer lifetime for the zwitterionic intermediate. 157
The [2 + 2] cycloaddition of ketenes and imines is an important route to the
-lactam ring (azetidinone), 158 which is a crucial structural feature of the penicillin
class of antibiotics. A number of theoretical treatments of this reaction indicate that in
solution phase this is a two-step reaction, with the second step being rate determining. 159
The stepwise nature of the reaction is accommodated by the relative stability of both
charged moieties, an iminium cation and an enolate anion.
154 E. J. Corey and M. C. Desai, Tetrahedron Lett., 26, 3535 (1985).
155 M. E. Kuehne and L. Foley, J. Org. Chem., 30, 4280 (1965).
156
J. K. Williams, D. W. Wiley, and B. C. McKusick, J. Am. Chem. Soc., 84, 2210 (1962).
157 R. Huisgen, Acc. Chem. Res., 10, 117, 199 (1977).
158 C. Palomo, J. M. Aizpurua, I. Ganboa, and M. Oiarbide, Eur. J. Org. Chem., 3223 (1999); I. Ojima and
F. Dalaloge, Chem. Soc. Rev., 26, 377 (1997).
159
X. Assfeld, J. A. Sordo, J. Gonzalez, M. F. Ruiz-Lopez, and T. L. Sordo, Theochem, 106, 193 (1993);
X. Assfeld, M. F. Ruiz-Lopez, J. Gonzalez, R. Lopez, J. A. Sordo, and T. L. Sordo, J. Comput. Chem.,
15, 479 (1994); T. N. Truong, J. Phys. Chem. B, 102, 7877 (1998).