Page 906 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 906
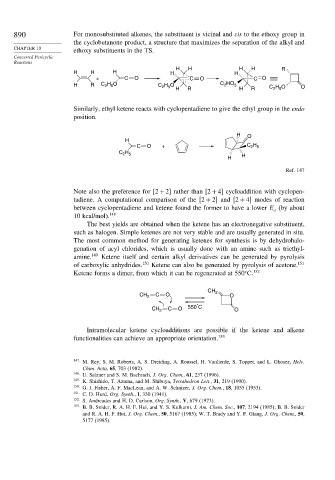
890 For monosubstituted alkenes, the substituent is vicinal and cis to the ethoxy group in
the cyclobutanone product, a structure that maximizes the separation of the alkyl and
CHAPTER 10
ethoxy substituents in the TS.
Concerted Pericyclic
Reactions
H H H H R
H H H H H
+ C O C O C O
H O
H O
H R C 2 5 C 2 5 C HO 5
2
H R H R C H O O
2 5
Similarly, ethyl ketene reacts with cyclopentadiene to give the ethyl group in the endo
position.
H O
H
C O + C H
2 5
C H
2 5
H H
Ref. 147
Note also the preference for 2 + 2 rather than 2 + 4 cycloaddition with cyclopen-
tadiene. A computational comparison of the 2 + 2 and 2 + 4 modes of reaction
between cyclopentadiene and ketene found the former to have a lower E (by about
a
10 kcal/mol). 148
The best yields are obtained when the ketene has an electronegative substituent,
such as halogen. Simple ketenes are not very stable and are usually generated in situ.
The most common method for generating ketenes for synthesis is by dehydrohalo-
genation of acyl chlorides, which is usually done with an amine such as triethyl-
amine. 149 Ketene itself and certain alkyl derivatives can be generated by pyrolysis
of carboxylic anhydrides. 150 Ketene can also be generated by pyrolysis of acetone. 151
Ketene forms a dimer, from which it can be regenerated at 550 C. 152
CH 2
CH 2 C O O
°
CH 2 C O 550 C O
Intramolecular ketene cycloadditions are possible if the ketene and alkene
functionalities can achieve an appropriate orientation. 153
147
M. Rey, S. M. Roberts, A. S. Dreiding, A. Roussel, H. Vanlierde, S. Toppet, and L. Ghosez, Helv.
Chim. Acta, 65, 703 (1982).
148 U. Salzner and S. M. Bachrach, J. Org. Chem., 61, 237 (1996).
149
K. Shishido, T. Azuma, and M. Shibuya, Tetrahedron Lett., 31, 219 (1990).
150
G. J. Fisher, A. F. MacLean, and A. W. Schnizer, J. Org. Chem., 18, 1055 (1953).
151 C. D. Hurd, Org. Synth., I, 330 (1941).
152 S. Andreades and H. D. Carlson, Org. Synth., V, 679 (1973).
153
B. B. Snider, R. A. H. F. Hui, and Y. S. Kulkarni, J. Am. Chem. Soc., 107, 2194 (1985); B. B. Snider
and R. A. H. F. Hui, J. Org. Chem., 50, 5167 (1985); W. T. Brady and Y. F. Giang, J. Org. Chem., 50,
5177 (1985).