Page 904 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 904
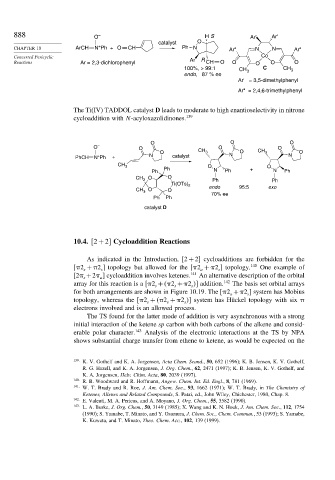
888 O – H S Ar' Ar'
catalyst O
+
CHAPTER 10 ArCH N Ph + O CH Ph N Ar" N N Ar"
Concerted Pericyclic Co
Reactions Ar = 2,3-dichlorophenyl Ar R CH O O O O O
100%, > 99:1 CH 3 C CH 3
endo, 87 % ee
Ar' = 3,5-dimethylphenyl
Ar" = 2,4,6-trimethylphenyl
The Ti(IV) TADDOL catalyst D leads to moderate to high enantioselectivity in nitrone
cycloaddition with N-acyloxazolidinones. 139
O O O
O – O O O
O CH 3 O CH 3 O
+
PhCH N Ph + N catalyst N N
CH 3 Ph O O
Ph N Ph + N Ph
O O
CH 3
Ph Ph
Ti(OTs) 2 endo 95:5 exo
O
CH 3 O
70% ee
Ph Ph
catalyst D
10.4. 2+2 Cycloaddition Reactions
As indicated in the Introduction, 2 + 2 cycloadditions are forbidden for the
[ 2 + 2 ] topology but allowed for the [ 2 + 2 ] topology. 140 One example of
s
a
s
s
[2 +2 ] cycloaddition involves ketenes. 141 An alternative description of the orbital
s a
array for this reaction is a [ 2 + 2 + 2 ] addition. 142 The basis set orbital arrays
s s s
for both arrangements are shown in Figure 10.19. The [ 2 + 2 ] system has Mobius
a
s
topology, whereas the [ 2 + 2 + 2 ] system has Hückel topology with six
s
s
s
electrons involved and is an allowed process.
The TS found for the latter mode of addition is very asynchronous with a strong
initial interaction of the ketene sp carbon with both carbons of the alkene and consid-
erable polar character. 143 Analysis of the electronic interactions at the TS by NPA
shows substantial charge transfer from ethene to ketene, as would be expected on the
139
K. V. Gothelf and K. A. Jorgensen, Acta Chem. Scand., 50, 652 (1996); K. B. Jensen, K. V. Gothelf,
R. G. Hazell, and K. A. Jorgensen, J. Org. Chem., 62, 2471 (1997); K. B. Jensen, K. V. Gothelf, and
K. A. Jorgensen, Helv. Chim. Acta, 80, 2039 (1997).
140 R. B. Woodward and R. Hoffmann, Angew. Chem. Int. Ed. Engl., 8, 781 (1969).
141
W. T. Brady and R. Roe, J. Am. Chem. Soc., 93, 1662 (1971); W. T. Brady, in The Chemistry of
Ketenes, Allenes and Related Compounds, S. Patai, ed., John Wiley, Chichester, 1980, Chap. 8.
142 E. Valenti, M. A. Pericas, and A. Moyano, J. Org. Chem., 55, 3582 (1990).
143
L. A. Burke, J. Org. Chem., 50, 3149 (1985); X. Wang and K. N. Houk, J. Am. Chem. Soc., 112, 1754
(1990); S. Yamabe, T. Minato, and Y. Osamura, J. Chem. Soc., Chem. Commun., 53 (1993); S. Yamabe,
K. Kuwata, and T. Minato, Theo. Chem. Acc., 102, 139 (1999).