Page 900 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 900
884 10.3.2. Scope and Applications of 1,3-Dipolar Cycloadditions
CHAPTER 10 As can be judged from Scheme 10.6, a wide variety of five-membered hetero-
Concerted Pericyclic cyclic compounds can be made by the 1,3-DPCA reaction. Sometimes, these products
Reactions are not the final target but rather intermediates for preparation of other compounds.
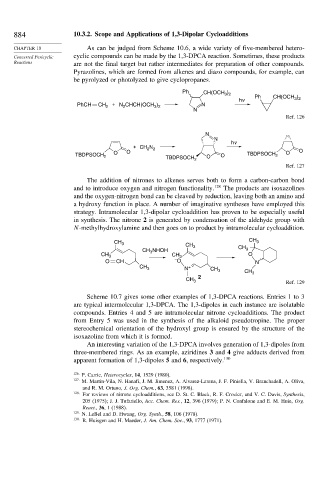
Pyrazolines, which are formed from alkenes and diazo compounds, for example, can
be pyrolyzed or photolyzed to give cyclopropanes.
Ph CH(OCH )
3 2
3 2
hv Ph CH(OCH )
PhCH CH 2 + N CHCH(OCH ) N
2
3 2
N
Ref. 126
N
N
hv
+ CH 2 N 2
O O O O
TBDPSOCH 2 O O TBDPSOCH 2
TBDPSOCH 2
Ref. 127
The addition of nitrones to alkenes serves both to form a carbon-carbon bond
and to introduce oxygen and nitrogen functionality. 128 The products are isoxazolines
and the oxygen-nitrogen bond can be cleaved by reduction, leaving both an amino and
a hydroxy function in place. A number of imaginative syntheses have employed this
strategy. Intramolecular 1,3-dipolar cycloaddition has proven to be especially useful
in synthesis. The nitrone 2 is generated by condensation of the aldehyde group with
N-methylhydroxylamine and then goes on to product by intramolecular cycloaddition.
CH 3 CH CH 3
CH NHOH 3 CH 3
CH 3 3 CH 3 O
O CH – O N
CH 3 N + CH 3 CH
2 3
CH 3 Ref. 129
Scheme 10.7 gives some other examples of 1,3-DPCA reactions. Entries 1 to 3
are typical intermolecular 1,3-DPCA. The 1,3-dipoles in each instance are isolatable
compounds. Entries 4 and 5 are intramolecular nitrone cycloadditions. The product
from Entry 5 was used in the synthesis of the alkaloid pseudotropine. The proper
stereochemical orientation of the hydroxyl group is ensured by the structure of the
isoxazoline from which it is formed.
An interesting variation of the 1,3-DPCA involves generation of 1,3-dipoles from
three-membered rings. As an example, aziridines 3 and 4 give adducts derived from
apparent formation of 1,3-dipoles 5 and 6, respectively. 130
126 P. Carrie, Heterocycles, 14, 1529 (1980).
127 M. Martin-Vila, N. Hanafi, J. M. Jimenez, A. Alvarez-Larena, J. F. Piniella, V. Branchadell, A. Oliva,
and R. M. Ortuno, J. Org. Chem., 63, 3581 (1998).
128
For reviews of nitrone cycloadditions, see D. St. C. Black, R. F. Crozier, and V. C. Davis, Synthesis,
205 (1975); J. J. Tufariello, Acc. Chem. Res., 12, 396 (1979); P. N. Confalone and E. M. Huie, Org.
React., 36, 1 (1988).
129 N. LeBel and D. Hwang, Org. Synth., 58, 106 (1978).
130
R. Huisgen and H. Maeder, J. Am. Chem. Soc., 93, 1777 (1971).