Page 901 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 901
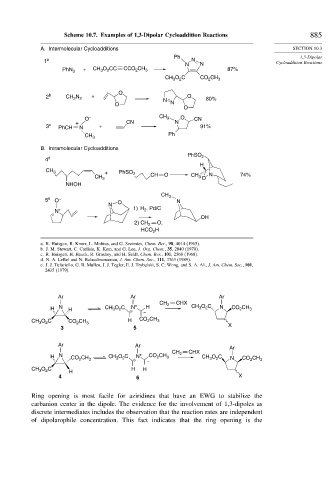
Scheme 10.7. Examples of 1,3-Dipolar Cycloaddition Reactions 885
A. Intermolecular Cycloadditions SECTION 10.3
Ph 1,3-Dipolar
1 a N Cycloaddition Reactions
N N
PhN 3 + CH O CC CCO CH 3 87%
2
3
2
CH O C CO CH 3
3
2
2
O
2 b CH N + N O 80%
2 2
O N
O
O – CH 3 O CN
+ CN N
3 c PhCH N + 91%
Ph
CH 3
B. Intramolecular Cycloadditions
PhSO 2
4 d
H
CH 3 + PhSO 2
CH 3 CH O CH 3 O N 74%
NHOH
CH 3
5 e O – O N
N
N + 1) H 2 , Pd/C
OH
2) CH 2 O,
HCO H
2
a. R. Huisgen, R. Knorr, L. Mobius, and G. Szeimies, Chem. Ber., 98, 4014 (1965).
b. J. M. Stewart, C. Carlisle, K. Kem, and G. Lee, J. Org. Chem., 35, 2040 (1970).
c. R. Huisgen, H. Hauck, R. Grashey, and H. Seidl, Chem. Ber., 101, 2568 (1968).
d. N. A. LeBel and N. Balasubramanian, J. Am. Chem. Soc., 111, 3363 (1989).
e. J. J. Tufariello, G. B. Mullen, J. J. Tegler, E. J. Trybulski, S. C. Wong, and S. A. Ali, J. Am. Chem. Soc., 101,
2435 (1979).
Ar Ar Ar
CH 2 CHX
H N H CH O C N + H CH O C N CO CH 3
3
2
2
2
3
–
O C CH H CO CH 3
2
CH 3 2 CO 2 3 X
3 5
Ar Ar Ar
CH 2 CHX
H N CO CH CH O C N + CO CH 3 CH O C N CO 2 CH
2
3
2
3
2
2
3
– 3
O C H
CH 3 2 H H
4 6 X
Ring opening is most facile for aziridines that have an EWG to stabilize the
carbanion center in the dipole. The evidence for the involvement of 1,3-dipoles as
discrete intermediates includes the observation that the reaction rates are independent
of dipolarophile concentration. This fact indicates that the ring opening is the