Page 897 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 897
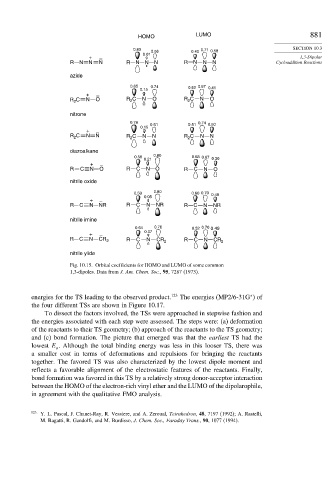
HOMO LUMO 881
0.83 0.56 0.40 0.71 0.58 SECTION 10.3
0.01
+ – 1,3-Dipolar
R N N N R N N N R N N N Cycloaddition Reactions
azide
0.65 0.74 0.62 0.67 0.41
0.15
+ –
R C N O R C N O R C N O
2
2
2
nitrone
0.78 0.51 0.74 0.50
0.15 0.61
+ –
R C N N R C N N R 2 C N N
2
2
diazoalkane
0.56 0.80 0.68 0.67
0.21 0.30
+ –
R C N O R C N O R C N O
nitrile oxide
0.59 0.80 0.60 0.70 0.40
0.05
+ –
R C N NR R C N NR R C N NR
nitrile imine
0.64 0.76 0.52 0.70 0.49
0.07
+ –
R C N CR 2 R C N CR 2 R C N CR 2
nitrile ylide
Fig. 10.15. Orbital coefficients for HOMO and LUMO of some common
1,3-dipoles. Data from J. Am. Chem. Soc., 95, 7287 (1973).
∗
energies for the TS leading to the observed product. 123 The energies (MP2/6-31G )of
the four different TSs are shown in Figure 10.17.
To dissect the factors involved, the TSs were approached in stepwise fashion and
the energies associated with each step were assessed. The steps were: (a) deformation
of the reactants to their TS geometry; (b) approach of the reactants to the TS geometry;
and (c) bond formation. The picture that emerged was that the earliest TS had the
lowest E . Although the total binding energy was less in this looser TS, there was
a
a smaller cost in terms of deformations and repulsions for bringing the reactants
together. The favored TS was also characterized by the lowest dipole moment and
reflects a favorable alignment of the electrostatic features of the reactants. Finally,
bond formation was favored in this TS by a relatively strong donor-acceptor interaction
between the HOMO of the electron-rich vinyl ether and the LUMO of the dipolarophile,
in agreement with the qualitative FMO analysis.
123
Y. L. Pascal, J. Chanet-Ray, R. Vessiere, and A. Zeroual, Tetrahedron, 48, 7197 (1992); A. Rastelli,
M. Bagatti, R. Gandolfi, and M. Burdisso, J. Chem. Soc., Faraday Trans., 90, 1077 (1994).