Page 909 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 909
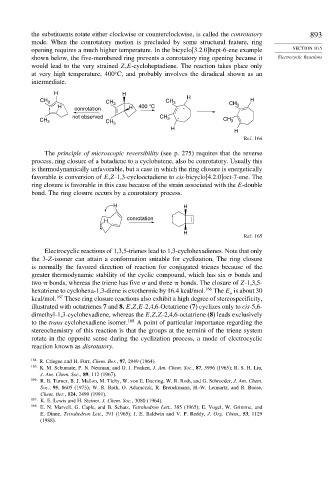
the substituents rotate either clockwise or counterclockwise, is called the conrotatory 893
mode. When the conrotatory motion is precluded by some structural feature, ring
opening requires a much higher temperature. In the bicyclo[3.2.0]hept-6-ene example SECTION 10.5
shown below, the five-membered ring prevents a conrotatory ring opening because it Electrocyclic Reactions
would lead to the very strained Z,E-cycloheptadiene. The reaction takes place only
at very high temperature, 400 C, and probably involves the diradical shown as an
intermediate.
H H
CH 3 CH CH 3 H H
H 3 H 400 °C . CH 3
conrotation
not observed CH 3 .
CH 3 CH 3 CH 3
H H
Ref. 164
The principle of microscopic reversibility (see p. 275) requires that the reverse
process, ring closure of a butadiene to a cyclobutene, also be conrotatory. Usually this
is thermodynamically unfavorable, but a case in which the ring closure is energetically
favorable is conversion of E,Z-1,3-cyclooctadiene to cis-bicyclo[4.2.0]oct-7-ene. The
ring closure is favorable in this case because of the strain associated with the E-double
bond. The ring closure occurs by a conrotatory process.
H H
conrotation
H
H
Ref. 165
Electrocyclic reactions of 1,3,5-trienes lead to 1,3-cyclohexadienes. Note that only
the 3-Z-isomer can attain a conformation suitable for cyclization. The ring closure
is normally the favored direction of reaction for conjugated trienes because of the
greater thermodynamic stability of the cyclic compound, which has six bonds and
two bonds, whereas the triene has five and three bonds. The closure of Z-1,3,5-
hexatriene to cyclohexa-1,3-diene is exothermic by 16.4 kcal/mol. 166 The E is about 30
a
kcal/mol. 167 These ring closure reactions also exhibit a high degree of stereospecificity,
illustrated with octatrienes 7 and 8. E,Z,E-2,4,6-Octatriene (7) cyclizes only to cis-5,6-
dimethyl-1,3-cyclohexadiene, whereas the E,Z,Z-2,4,6-octatriene (8) leads exclusively
to the trans cyclohexadiene isomer. 168 A point of particular importance regarding the
stereochemistry of this reaction is that the groups at the termini of the triene system
rotate in the opposite sense during the cyclization process, a mode of electrocyclic
reaction known as disrotatory.
164 R. Criegee and H. Furr, Chem. Ber., 97, 2949 (1964).
165
K. M. Schumate, P. N. Neuman, and G. J. Fonken, J. Am. Chem. Soc., 87, 3996 (1965); R. S. H. Liu,
J. Am. Chem. Soc., 89, 112 (1967).
166 R. B. Turner, B. J. Mallon, M. Tichy, W. von E. Doering, W. R. Roth, and G. Schroeder, J. Am. Chem.
Soc., 95, 8605 (1973); W. R. Roth, O. Adamczak, R. Breuckmann, H.-W. Lennartz, and R. Boese,
Chem. Ber., 124, 2499 (1991).
167 K. E. Lewis and H. Steiner, J. Chem. Soc., 3080 (1964).
168
E. N. Marvell, G. Caple, and B. Schatz, Tetrahedron Lett., 385 (1965); E. Vogel, W. Grimme, and
E. Dinne, Tetrahedron Lett., 391 (1965); J. E. Baldwin and V. P. Reddy, J. Org. Chem., 53, 1129
(1988).