Page 915 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 915
899
SECTION 10.5
Electrocyclic Reactions
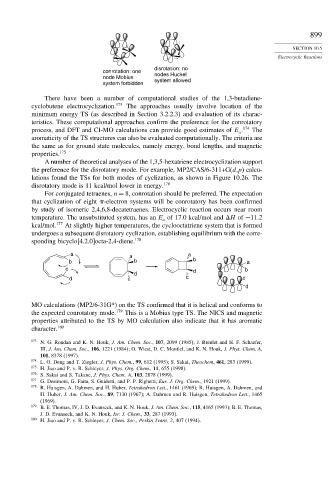
disrotation: no
conrotation: one nodes Huckel
node Mobius system allowed
system forbidden
There have been a number of computational studies of the 1,3-butadiene-
cyclobutene electrocyclization. 173 The approaches usually involve location of the
minimum energy TS (as described in Section 3.2.2.3) and evaluation of its charac-
teristics. These computational approaches confirm the preference for the conrotatory
process, and DFT and CI-MO calculations can provide good estimates of E . 174 The
a
aromaticity of the TS structures can also be evaluated computationally. The criteria are
the same as for ground state molecules, namely energy, bond lengths, and magnetic
properties. 175
A number of theoretical analyses of the 1,3,5-hexatriene electrocyclization support
the preference for the disrotatory mode. For example, MP2/CAS/6-311+G(d,p calcu-
lations found the TSs for both modes of cyclization, as shown in Figure 10.26. The
disrotatory mode is 11 kcal/mol lower in energy. 176
For conjugated tetraenes, n = 8, conrotation should be preferred. The expectation
that cyclization of eight -electron systems will be conrotatory has been confirmed
by study of isomeric 2,4,6,8-decatetraenes. Electrocyclic reaction occurs near room
temperature. The unsubstituted system, has an E of 17.0 kcal/mol and H of −11 2
a
kcal/mol. 177 At slightly higher temperatures, the cyclooctatriene system that is formed
undergoes a subsequent disrotatory cyclization, establishing equilibrium with the corre-
sponding bicyclo[4.2.0]octa-2,4-diene. 178
a a a
b b b a
c d b
d
d c c c
d
MO calculations (MP2/6-31G*) on the TS confirmed that it is helical and conforms to
the expected conrotatory mode. 179 This is a Mobius type TS. The NICS and magnetic
properties attributed to the TS by MO calculation also indicate that it has aromatic
character. 180
173
N. G. Rondan and K. N. Houk, J. Am. Chem. Soc., 107, 2099 (1985); J. Breulet and H. F. Schaefer,
III, J. Am. Chem. Soc., 106, 1221 (1984); O. Wiest, D. C. Montiel, and K. N. Houk, J. Phys. Chem. A,
101, 8378 (1997).
174 L. O. Deng and T. Ziegler, J. Phys. Chem., 99, 612 (1995); S. Sakai, Theochem, 461, 283 (1999).
175
H. Jiao and P. v. R. Schleyer, J. Phys. Org. Chem., 11, 655 (1998).
176 S. Sakai and S. Takane, J. Phys. Chem. A, 103, 2878 (1999).
177 G. Desimoni, G. Faita, S. Guidetti, and P. P. Righetti, Eur. J. Org. Chem., 1921 (1999).
178
R. Huisgen, A. Dahmen, and H. Huber, Tetrahedron Lett., 1461 (1969); R. Huisgen, A. Dahmen, and
H. Huber, J. Am. Chem. Soc., 89, 7130 (1967); A. Dahmen and R. Huisgen, Tetrahedron Lett., 1465
(1969).
179 B. E. Thomas, IV, J. D. Evanseck, and K. N. Houk, J. Am. Chem. Soc., 115, 4165 (1993); B. E. Thomas,
J. D. Evanseck, and K. N. Houk, Isr. J. Chem., 33, 287 (1993).
180
H. Jiao and P. v. R. Schleyer, J. Chem. Soc., Perkin Trans. 2, 407 (1994).