Page 919 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 919
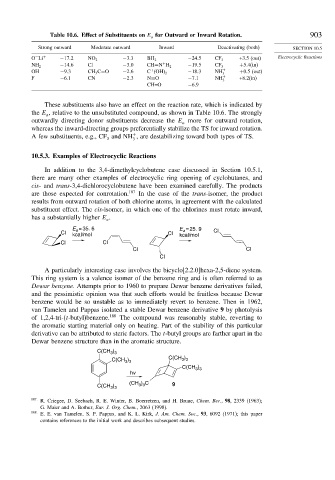
Table 10.6. Effect of Substituents on E for Outward or Inward Rotation. 903
a
Strong outward Moderate outward Inward Deactivating (both) SECTION 10.5
O Li + −17 2 NO 2 −3 3 BH 2 −24 5 CF 3 +3.5 (out) Electrocyclic Reactions
−
−14 6 Cl −3 0 + −19 5 +5 4(in)
NH 2 CH=N H 2 CF 3
OH −9 3 CH 3 C=O −2 6 C (OH) 2 −18 3 NH + +0 5 (out)
+
3
F −6 1 CN −2 3 N=O −7 1 NH + +8.2(in)
3
CH=O −6 9
These substituents also have an effect on the reaction rate, which is indicated by
the E , relative to the unsubstituted compound, as shown in Table 10.6. The strongly
a
outwardly directing donor substituents decrease the E more for outward rotation,
a
whereas the inward-directing groups preferentially stabilize the TS for inward rotation.
+
A few substituents, e.g., CF and NH , are destabilizing toward both types of TS.
3
3
10.5.3. Examples of Electrocyclic Reactions
In addition to the 3,4-dimethylcyclobutene case discussed in Section 10.5.1,
there are many other examples of electrocyclic ring opening of cyclobutanes, and
cis- and trans-3,4-dichlorocyclobutene have been examined carefully. The products
are those expected for conrotation. 187 In the case of the trans-isomer, the product
results from outward rotation of both chlorine atoms, in agreement with the calculated
substituent effect. The cis-isomer, in which one of the chlorines must rotate inward,
has a substantially higher E .
a
E a = 35. 6 E = 25. 9 Cl
a
Cl kcal/mol Cl kcal/mol
Cl Cl
Cl Cl
Cl
A particularly interesting case involves the bicyclo[2.2.0]hexa-2,5-diene system.
This ring system is a valence isomer of the benzene ring and is often referred to as
Dewar benzene. Attempts prior to 1960 to prepare Dewar benzene derivatives failed,
and the pessimistic opinion was that such efforts would be fruitless because Dewar
benzene would be so unstable as to immediately revert to benzene. Then in 1962,
van Tamelen and Pappas isolated a stable Dewar benzene derivative 9 by photolysis
of 1,2,4-tri-(t-butyl)benzene. 188 The compound was reasonably stable, reverting to
the aromatic starting material only on heating. Part of the stability of this particular
derivative can be attributed to steric factors. The t-butyl groups are farther apart in the
Dewar benzene structure than in the aromatic structure.
C(CH )
3 3
C(CH ) C(CH 3 ) 3
3 3
C(CH )
3 3
hv
(CH 3 ) 3 C 9
C(CH 3 ) 3
187 R. Criegee, D. Seebach, R. E. Winter, B. Boerretzen, and H. Brune, Chem. Ber., 98, 2339 (1963);
G. Maier and A. Bothur, Eur. J. Org. Chem., 2063 (1998).
188
E. E. van Tamelen. S. P. Pappas, and K. L. Kirk, J. Am. Chem. Soc., 93, 6092 (1971); this paper
contains references to the initial work and describes subsequent studies.