Page 923 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 923
possible case of an electrocyclic process because it involves only two electrons. 197 907
Owing to the strain imposed by the cyclopropyl ring, cyclopropyl cations do not form
easily, and cyclopropyl halides and sulfonates are quite unreactive under ordinary SECTION 10.5
solvolytic conditions. For example, solvolysis of cyclopropyl tosylate in acetic acid Electrocyclic Reactions
requires a temperature of 180 C. The product is allyl acetate rather than cyclopropyl
acetate. 198 This transformation might occur by formation of the cyclopropyl cation,
followed by ring opening to the allyl cation or (see below) the reaction might occur
in a single step.
X -X - + fast
+
slow H
H
Formation of allylic products is characteristic of solvolytic reaction of other cyclo-
propyl halides and sulfonates. Similarly, diazotization of cyclopropylamine in aqueous
solution gives allyl alcohol. 199
The ring opening of a cyclopropyl cation is an electrocyclic process of the
4n + 2 type, where n equals zero, and should therefore be a disrotatory process.
CCSD(T)/6-311G(2d) and B3LYP/6-31G(2d) computations on the reaction indicate
that the cyclopropyl cation is not a stable intermediate and that there is no barrier
to electrocyclic ring opening. 200 This result implies that the ring opening occurs as a
concerted process in conjunction with rupture of the bond to the leaving group.
As with cyclobutenes, there are two possible directions for the allowed disro-
tation in substituted cyclopropyl cations. For a cis-2,3-dimethylcyclopropyl cation, for
example, two different disrotatory modes are possible, leading to structurally distinct
allyl cations.
CH 3
CH 3 CH 3
CH 3
CH 3 + CH 3 +
+ CH 3 +
CH 3
inward rotation U-shaped cation outward rotation W-shaped cation
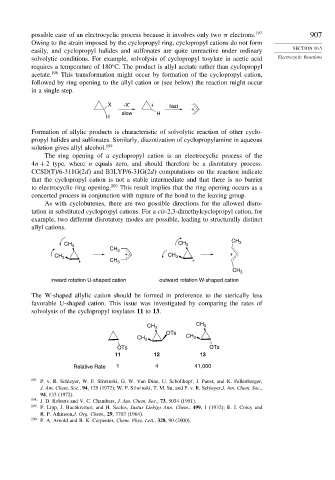
The W-shaped allylic cation should be formed in preference to the sterically less
favorable U-shaped cation. This issue was investigated by comparing the rates of
solvolysis of the cyclopropyl tosylates 11 to 13.
CH 3 CH 3
OTs
CH 3 CH 3
OTs OTs
11 12 13
Relative Rate 1 4 41,000
197 P. v. R. Schleyer, W. F. Sliwinski, G. W. Van Dine, U. Schollkopf, J. Paust, and K. Fellenberger,
J. Am. Chem. Soc., 94, 125 (1972); W. F. Sliwinski, T. M. Su, and P. v. R. Schleyer,J. Am. Chem. Soc.,
94, 133 (1972).
198
J. D. Roberts and V. C. Chambers, J. Am. Chem. Soc., 73, 5034 (1951).
199 P. Lipp, J. Buchkremer, and H. Seeles, Justus Liebigs Ann. Chem., 499, 1 (1932); E. J. Corey and
R. F. Atkinson,J. Org. Chem., 29, 3703 (1964).
200
P. A. Arnold and B. K. Carpenter, Chem. Phys. Lett., 328, 90 (2000).