Page 926 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 926
−1
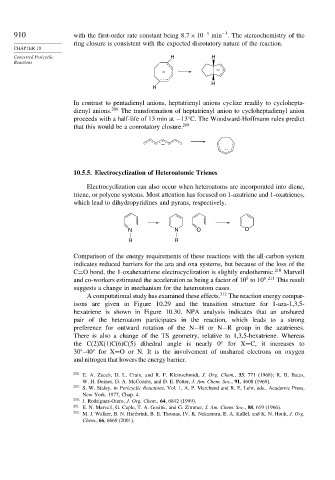
910 with the first-order rate constant being 8 7×10 −3 min . The stereochemistry of the
ring closure is consistent with the expected disrotatory nature of the reaction.
CHAPTER 10
Concerted Pericyclic H H
Reactions
–
–
H
H
In contrast to pentadienyl anions, heptatrienyl anions cyclize readily to cyclohepta-
dienyl anions. 208 The transformation of heptatrienyl anion to cycloheptadienyl anion
proceeds with a half-life of 13 min at −13 C. The Woodward-Hoffmann rules predict
that this would be a conrotatory closure. 209
–
–
10.5.5. Electrocyclization of Heteroatomic Trienes
Electrocyclization can also occur when heteroatoms are incorporated into diene,
triene, or polyene systems. Most attention has focused on 1-azatriene and 1-oxatrienes,
which lead to dihydropyridines and pyrans, respectively.
N N O O
R R
Comparison of the energy requirements of these reactions with the all-carbon system
indicates reduced barriers for the aza and oxa systems, but because of the loss of the
C=O bond, the 1-oxahexatriene electrocyclization is slightly endothermic. 210 Marvell
5
6 211
and co-workers estimated the acceleration as being a factor of 10 to 10 . This result
suggests a change in mechanism for the heteroatom cases.
A computational study has examined these effects. 212 The reaction energy compar-
isons are given in Figure 10.29 and the transition structure for 1-aza-1,3,5-
hexatriene is shown in Figure 10.30. NPA analysis indicates that an unshared
pair of the heteroatom participates in the reaction, which leads to a strong
preference for outward rotation of the N−HorN−R group in the azatrienes.
There is also a change of the TS geometry, relative to 1,3,5-hexatriene. Whereas
the C(2)X(1)C(6)C(5) dihedral angle is nearly 0 for X=C, it increases to
30 –40 for X=O or N. It is the involvement of unshared electrons on oxygen
and nitrogen that lowers the energy barrier.
208 E. A. Zuech, D. L. Crain, and R. F. Kleinschmidt, J. Org. Chem., 33, 771 (1968); R. B. Bates,
W. H. Deines, D. A. McCombs, and D. E. Potter, J. Am. Chem. Soc., 91, 4608 (1969).
209 S. W. Staley, in Pericyclic Reactions, Vol. 1, A. P. Marchand and R. E. Lehr, eds., Academic Press,
New York, 1977, Chap. 4.
210
J. Rodriguez-Otero, J. Org. Chem., 64, 6842 (1999).
211 E. N. Marvell, G. Caple, T. A. Gosink, and G. Zimmer, J. Am. Chem. Soc., 88, 619 (1966).
212
M. J. Walker, B. N. Hietbrink, B. E. Thomas, IV, K. Nakamura, E. A. Kallel, and K. N. Houk, J. Org.
Chem., 66, 6669 (2001).