Page 929 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 929
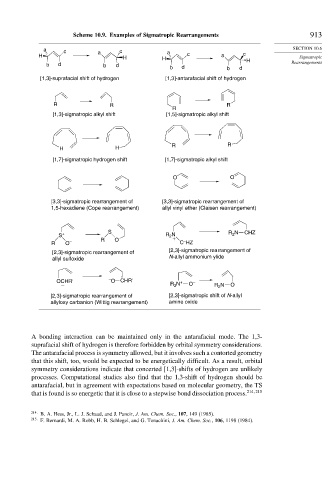
Scheme 10.9. Examples of Sigmatropic Rearrangements 913
a c a c a SECTION 10.6
H H H c a c H Sigmatropic
b d b d b d b d Rearrangements
[1,3]-suprafacial shift of hydrogen [1,3]-antarafacial shift of hydrogen
R R R
R
[1,3]-sigmatropic alkyl shift [1,5]-sigmatropic alkyl shift
R R
H H
[1,7]-sigmatropic hydrogen shift [1,7]-sigmatropic alkyl shift
O O
[3,3]-sigmatropic rearrangement of [3,3]-sigmatropic rearrangement of
1,5-hexadiene (Cope rearrangement) allyl vinyl ether (Claisen rearrangement)
S R N CHZ
S + R N 2
2
R O + –
R O – C HZ
[2,3]-sigmatropic rearrangement of [2,3]-sigmatropic rearrangement of
allyl sulfoxide N-allyl ammonium ylide
OCHR' – O CHR' + –
– R N O R N O
2
2
[2,3]-sigmatropic rearrangement of [2,3]-sigmatropic shift of N-allyl
allyloxy carbanion (Wittig rearrangement) amine oxide
A bonding interaction can be maintained only in the antarafacial mode. The 1,3-
suprafacial shift of hydrogen is therefore forbidden by orbital symmetry considerations.
The antarafacial process is symmetry allowed, but it involves such a contorted geometry
that this shift, too, would be expected to be energetically difficult. As a result, orbital
symmetry considerations indicate that concerted [1,3]-shifts of hydrogen are unlikely
processes. Computational studies also find that the 1,3-shift of hydrogen should be
antarafacial, but in agreement with expectations based on molecular geometry, the TS
that is found is so energetic that it is close to a stepwise bond dissociation process. 214 215
214 B. A. Hess, Jr., L. J. Schaad, and J. Pancir, J. Am. Chem. Soc., 107, 149 (1985).
215
F. Bernardi, M. A. Robb, H. B. Schlegel, and G. Tonachini, J. Am. Chem. Soc., 106, 1198 (1984).