Page 934 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 934
918 CH 3
CH 3
CHAPTER 10 CH 3 H
Concerted Pericyclic CH 3
Reactions CH 3
TS11'
H 1' 1 H CH 3
CH 3 35.3 TS23 TS34 TS12
TS1'2' TS11' TS12 32.7
33.0 32.7
2
2' CH 3 CH 3
H
H
3.4 3 3.1 4
TS2'3' 1.1 1' 1.1 1 0.0 TS23
CH 3 2
CH 3
CH 3 CH 3
3' CH 3
3
H H
TS3'4
TS34
4
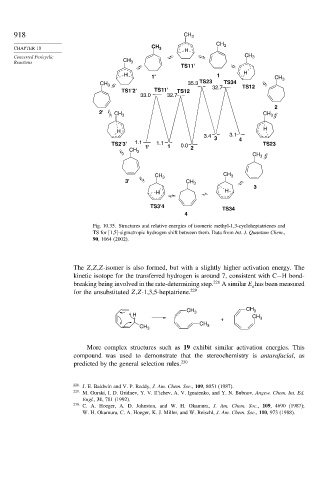
Fig. 10.35. Structures and relative energies of isomeric methyl-1,3-cycloheptatrienes and
TS for [1,5]-sigmatropic hydrogen shift between them. Data from Int. J. Quantum Chem.,
90, 1064 (2002).
The Z,Z,Z-isomer is also formed, but with a slightly higher activation energy. The
kinetic isotope for the transferred hydrogen is around 7, consistent with C−H bond-
breaking being involved in the rate-determining step. 228 A similar E has been measured
a
for the unsubstituted Z,Z-1,3,5-heptatriene. 229
CH 3 CH 3
H CH
+ 3
CH
CH 3 3
More complex structures such as 19 exhibit similar activation energies. This
compound was used to demonstrate that the stereochemistry is antarafacial,as
predicted by the general selection rules. 230
228
J. E. Baldwin and V. P. Reddy, J. Am. Chem. Soc., 109, 8051 (1987).
229 M. Gurski, I. D. Gridnev, Y. V. Il’ichev, A. V. Ignatenko, and Y. N. Bubnov, Angew. Chem. Int. Ed.
Engl., 31, 781 (1992).
230
C. A. Hoeger, A. D. Johnston, and W. H. Okamura, J. Am. Chem. Soc., 109, 4690 (1987);
W. H. Okamura, C. A. Hoeger, K. J. Miller, and W. Reischl, J. Am. Chem. Soc., 110, 973 (1988).