Page 935 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 935
CH 3 C H CH 3 C H 919
8 17
CH 3 C H 8 17
8 17
SECTION 10.6
D + H CH Sigmatropic
CH H CH 3 Rearrangements
3
OH 3 OH D OH
D
H
H H
19
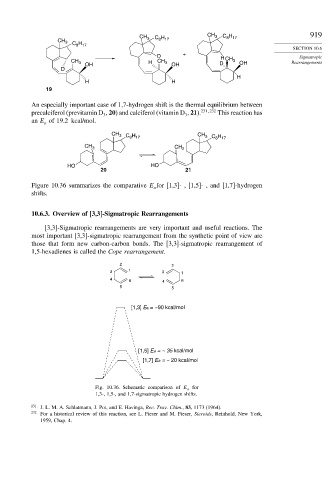
An especially important case of 1,7-hydrogen shift is the thermal equilibrium between
precalciferol (previtamin D , 20) and calciferol (vitamin D , 21). 231 232 This reaction has
3
3
an E of 19.2 kcal/mol.
a
CH 3 C H 17 CH 3 C H
8
8 17
CH 3 CH 2
HO HO
20 21
Figure 10.36 summarizes the comparative E for [1,3]- , [1,5]- , and [1,7]-hydrogen
a
shifts.
10.6.3. Overview of [3,3]-Sigmatropic Rearrangements
[3,3]-Sigmatropic rearrangements are very important and useful reactions. The
most important [3,3]-sigmatropic rearrangement from the synthetic point of view are
those that form new carbon-carbon bonds. The [3,3]-sigmatropic rearrangement of
1,5-hexadienes is called the Cope rearrangement.
2 2
3 1 3 1
4 6 4 6
5 5
[1,3] Ea = ~90 kcal/mol
[1,5] Ea = ~ 35 kcal/mol
[1,7] Ea = ~ 20 kcal/mol
Fig. 10.36. Schematic comparison of E a for
1,3-, 1,5-, and 1,7-sigmatropic hydrogen shifts.
231 J. L. M. A. Schlatmann, J. Pot, and E. Havinga, Rec. Trav. Chim., 83, 1173 (1964).
232
For a historical review of this reaction, see L. Fieser and M. Fieser, Steroids, Reinhold, New York,
1959, Chap. 4.