Page 938 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 938
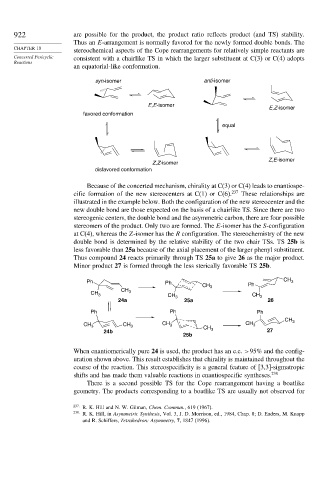
922 are possible for the product, the product ratio reflects product (and TS) stability.
Thus an E-arrangement is normally favored for the newly formed double bonds. The
CHAPTER 10
stereochemical aspects of the Cope rearrangements for relatively simple reactants are
Concerted Pericyclic consistent with a chairlike TS in which the larger substituent at C(3) or C(4) adopts
Reactions
an equatorial-like conformation.
syn-isomer anti-isomer
E,E-isomer
E,Z-isomer
favored conformation
equal
Z,E-isomer
Z,Z-isomer
disfavored conformation
Because of the concerted mechanism, chirality at C(3) or C(4) leads to enantiospe-
cific formation of the new stereocenters at C(1) or C(6). 237 These relationships are
illustrated in the example below. Both the configuration of the new stereocenter and the
new double bond are those expected on the basis of a chairlike TS. Since there are two
stereogenic centers, the double bond and the asymmetric carbon, there are four possible
stereomers of the product. Only two are formed. The E-isomer has the S-configuration
at C(4), whereas the Z-isomer has the R configuration. The stereochemistry of the new
double bond is determined by the relative stability of the two chair TSs. TS 25b is
less favorable than 25a because of the axial placement of the larger phenyl substituent.
Thus compound 24 reacts primarily through TS 25a to give 26 as the major product.
Minor product 27 is formed through the less sterically favorable TS 25b.
Ph Ph Ph CH 3
CH 3
CH
CH 3 3 CH 3 CH 3
24a 25a 26
Ph Ph Ph
CH 3
CH 3 CH 3 CH 3 CH CH 3
24b 3 27
25b
When enantiomerically pure 24 is used, the product has an e.e. >95% and the config-
uration shown above. This result establishes that chirality is maintained throughout the
course of the reaction. This stereospecificity is a general feature of [3,3]-sigmatropic
shifts and has made them valuable reactions in enantiospecific syntheses. 238
There is a second possible TS for the Cope rearrangement having a boatlike
geometry. The products corresponding to a boatlike TS are usually not observed for
237 R. K. Hill and N. W. Gilman, Chem. Commun., 619 (1967).
238
R. K. Hill, in Asymmetric Synthesis, Vol. 3, J. D. Morrison, ed., 1984, Chap. 8; D. Enders, M. Knopp
and R. Schiffers, Tetrahedron: Asymmetry, 7, 1847 (1996).