Page 937 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 937
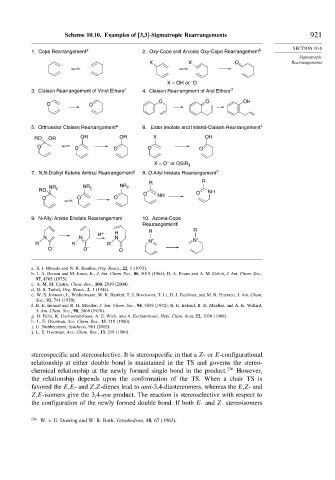
Scheme 10.10. Examples of [3,3]-Sigmatropic Rearrangements 921
SECTION 10.6
1. Cope Rearrangement a 2. Oxy-Cope and Anionic Oxy-Cope Rearrangement b
Sigmatropic
X X O Rearrangements
–
X = OH or O
3. Claisen Rearrangement of Vinyl Ethers c 4. Claisen Rearrangment of Aryl Ethers d
O O OH
O O
5. Orthoester Claisen Rearrangement e 6. Ester enolate and Ireland-Claisen Rearrangement f
RO OR OR OR X OH
O O O O O
–
X = O or OSiR 3
7. N,N-Dialkyl Ketene Aminal Rearrangement g 8. O-Allyl Imidate Rearrangement h
R R
RO NR 2 NR 2 NR 2 NH
O NH O
O O O
9. N-Allyl Amide Enolate Rearrangement 10. Azonia-Cope
Rearrangement j
H + H R R
N N N +
N + N
R R R
O – O – O
a. S. J. Rhoads and N. R. Raulins, Org. React., 22, 1 (1975).
b. J. A. Berson and M. Jones, Jr., J. Am. Chem. Soc., 86, 5019 (1964); D. A. Evans and A. M. Golob, J. Am. Chem. Soc.,
97, 4765 (1975).
c. A. M. M. Castro, Chem. Rev., 104, 2939 (2004).
d. D. S. Tarbel, Org. React., 2, 1 (1944).
e. W. S. Johnson, L. Wethermann, W. R. Bartlett, T. J. Brocksom, T. Li, D. J. Faulkner, and M. R. Petersen, J. Am. Chem.
Soc., 92, 741 (1970).
f. R. E. Ireland and R. H. Mueller, J. Am. Chem. Soc., 94, 5898 (1972); R. E. Ireland, R. H. Mueller, and A. K. Willard,
J. Am. Chem. Soc., 98, 2868 (1976).
g. D. Felix, K. Gschwend-Steen, A. E. Wick, and A. Eschenmoser, Helv. Chim. Acta, 52, 1030 (1969).
h. L. E. Overman, Acc. Chem. Res., 13, 218 (1980).
i. U. Nubbemeyer, Synthesis, 961 (2003).
j. L. E. Overman, Acc. Chem. Res., 13, 218 (1980).
stereospecific and stereoselective. It is stereospecific in that a Z-or E-configurational
relationship at either double bond is maintained in the TS and governs the stereo-
chemical relationship at the newly formed single bond in the product. 236 However,
the relationship depends upon the conformation of the TS. When a chair TS is
favored the E,E- and Z,Z-dienes lead to anti-3,4-diastereomers, whereas the E,Z- and
Z,E-isomers give the 3,4-syn product. The reaction is stereoselective with respect to
the configuration of the newly formed double bond. If both E- and Z- stereoisomers
236
W. v. E. Doering and W. R. Roth, Tetrahedron, 18, 67 (1962).