Page 939 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 939
acyclic dienes. However, this TS is allowed by orbital symmetry rules and if steric 923
factors make a boat TS preferable to a chair, reaction proceeds through a boat.
SECTION 10.6
Sigmatropic
Rearrangements
It is generally agreed that the boat TS is higher in energy than the chair TS. There
have been several studies aimed at determining the energy difference between the two.
One study involved 1,1,1,8,8,8-deuterio-4,5-dimethyloctadienes. The chair and boat
TSs predict different stereoisomeric products.
H
H H
H
CH 3
CH 3
CH 3 CH 3
CD 3 CD 3
CH 3 CD 3 CH 3 CD 3 CH 3 CD 3 CH 3 CD 3
H
H CD 3 H CD 3
H
chair syn boat anti
Although the process is further complicated by cis-trans isomerizations not considered
in the above structures, by analysis of the product ratio it was possible to determine
that the boat TS is about 6 kcal/mol less stable than the chair. 239 Related experiments
‡
on deuterated 1,5-hexadiene itself indicated a difference of 5.8 kcal in G for the
chair and boat TSs. 240
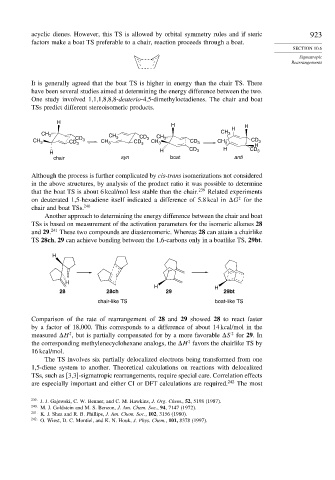
Another approach to determining the energy difference between the chair and boat
TSs is based on measurement of the activation parameters for the isomeric alkenes 28
and 29. 241 These two compounds are diastereomeric. Whereas 28 can attain a chairlike
TS 28ch, 29 can achieve bonding between the 1,6-carbons only in a boatlike TS, 29bt.
H
H
H H
28 28ch 29 29bt
chair-like TS boat-like TS
Comparison of the rate of rearrangement of 28 and 29 showed 28 to react faster
by a factor of 18,000. This corresponds to a difference of about 14 kcal/mol in the
‡
‡
measured H , but is partially compensated for by a more favorable S for 29.In
‡
the corresponding methylenecyclohexane analogs, the H favors the chairlike TS by
16 kcal/mol.
The TS involves six partially delocalized electrons being transformed from one
1,5-diene system to another. Theoretical calculations on reactions with delocalized
TSs, such as [3,3]-sigmatropic rearrangements, require special care. Correlation effects
are especially important and either CI or DFT calculations are required. 242 The most
239
J. J. Gajewski, C. W. Benner, and C. M. Hawkins, J. Org. Chem., 52, 5198 (1987).
240
M. J. Goldstein and M. S. Benzon, J. Am. Chem. Soc., 94, 7147 (1972).
241 K. J. Shea and R. B. Phillips, J. Am. Chem. Soc., 102, 3156 (1980).
242
O. Wiest, D. C. Montiel, and K. N. Houk, J. Phys. Chem., 101, 8378 (1997).