Page 944 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 944
928
CHAPTER 10
Concerted Pericyclic +63.5 20.1
Reactions
+53.6
C(3)–C(4) Bond-breaking
2
20.1 +79.7
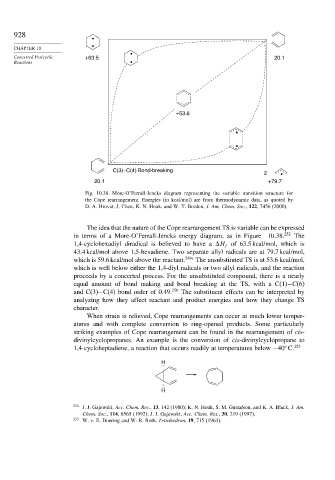
Fig. 10.38. More-O’Ferrall-Jencks diagram representing the variable transition structure for
the Cope rearrangement. Energies (in kcal/mol) are from thermodynamic data, as quoted by
D. A. Hrovat, J. Chen, K. N. Houk, and W. T. Borden, J. Am. Chem. Soc., 122, 7456 (2000).
The idea that the nature of the Cope rearrangement TS is variable can be expressed
in terms of a More-O’Ferrall-Jencks energy diagram, as in Figure 10.38. 252 The
1,4-cyclohexadiyl diradical is believed to have a H of 63.5 kcal/mol, which is
f
43.4 kcal/mol above 1,5-hexadiene. Two separate allyl radicals are at 79.7 kcal/mol,
which is 59.6 kcal/mol above the reactant. 249a The unsubstituted TS is at 53.6 kcal/mol,
which is well below either the 1,4-diyl radicals or two allyl radicals, and the reaction
proceeds by a concerted process. For the unsubstituted compound, there is a nearly
equal amount of bond making and bond breaking at the TS, with a C(1)−C(6)
and C(3)−C(4) bond order of 0.49. 250 The substituent effects can be interpreted by
analyzing how they affect reactant and product energies and how they change TS
character.
When strain is relieved, Cope rearrangements can occur at much lower temper-
atures and with complete conversion to ring-opened products. Some particularly
striking examples of Cope rearrangement can be found in the rearrangement of cis-
divinylcyclopropanes. An example is the conversion of cis-divinylcyclopropane to
1,4-cycloheptadiene, a reaction that occurs readily at temperatures below −40 C. 253
H
H
252 J. J. Gajewski, Acc. Chem. Res., 13, 142 (1980); K. N. Houk, S. M. Gustafson, and K. A. Black, J. Am.
Chem. Soc., 114, 8565 (1992); J. J. Gajewski, Acc. Chem. Res., 30, 219 (1997).
253
W. v. E. Doering and W. R. Roth, Tetrahedron, 19, 715 (1963).