Page 947 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 947
diagram below by the interchanging environments of the labeled carbons. The E for 931
a
the rearrangement has been determined to be 13.9 kcal/mol. 266 Substituted bullvalenes
have also been studied. 267 SECTION 10.6
Sigmatropic
Rearrangements
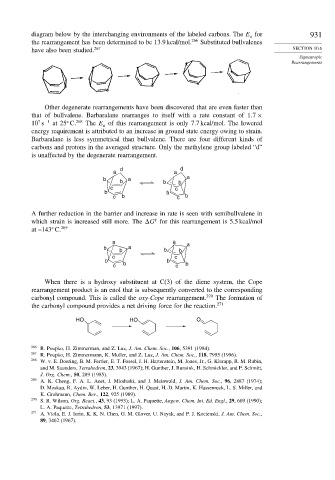
Other degenerate rearrangements have been discovered that are even faster than
that of bullvalene. Barbaralane rearranges to itself with a rate constant of 1 7 ×
7 −1
10 s at 25 C. 268 The E of this rearrangement is only 7.7 kcal/mol. The lowered
a
energy requirement is attributed to an increase in ground state energy owing to strain.
Barbaralane is less symmetrical than bullvalene. There are four different kinds of
carbons and protons in the averaged structure. Only the methylene group labeled “d”
is unaffected by the degenerate rearrangement.
d d
a a
a
b a
b b b
c c
b b
c b c b
A further reduction in the barrier and increase in rate is seen with semibullvalene in
‡
which strain is increased still more. The G for this rearrangement is 5.5 kcal/mol
at –143 C. 269
a a a
b a
b b b
c c
b b
c b c b
When there is a hydroxy substituent at C(3) of the diene system, the Cope
rearrangement product is an enol that is subsequently converted to the corresponding
carbonyl compound. This is called the oxy-Cope rearrangement. 270 The formation of
the carbonyl compound provides a net driving force for the reaction. 271
HO HO O
266
R. Poupko, H. Zimmerman, and Z. Luz, J. Am. Chem. Soc., 106, 5391 (1984).
267 R. Poupko, H. Zimmermann, K. Muller, and Z. Luz, J. Am. Chem. Soc., 118, 7995 (1996).
268 W. v. E. Doering, B. M. Ferrier, E. T. Fossel, J. H. Hartenstein, M. Jones, Jr., G. Klumpp, R. M. Rubin,
and M. Saunders, Tetrahedron, 23, 3943 (1967); H. Gunther, J. Runsink, H. Schmickler, and P. Schmitt,
J. Org. Chem., 50, 289 (1985).
269
A. K. Cheng, F. A. L. Anet, J. Mioduski, and J. Meinwald, J. Am. Chem. Soc., 96, 2887 (1974);
D. Moskau, R. Aydin, W. Leber, H. Gunther, H. Quast, H.-D. Martin, K. Hassenruck, L. S. Miller, and
K. Grohmann, Chem. Ber., 122, 925 (1989).
270 S. R. Wilson, Org. React., 43, 93 (1993); L. A. Paquette, Angew. Chem. Int. Ed. Engl., 29, 609 (1990);
L. A. Paquette, Tetrahedron, 53, 13971 (1997).
271
A. Viola, E. J. Iorio, K. K. N. Chen, G. M. Glover, U. Nayak, and P. J. Kocienski, J. Am. Chem. Soc.,
89, 3462 (1967).